T31003
Thiolactic acid
95%
Synonym(s):
2-Mercaptopropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
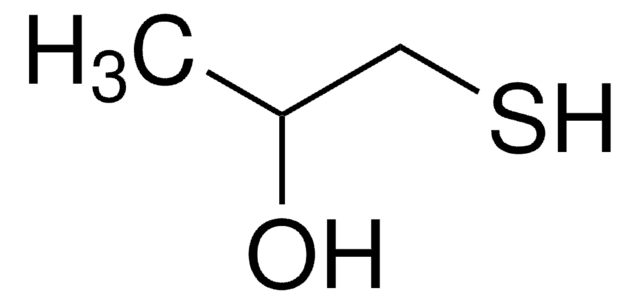
CH3CH(SH)COOH
CAS Number:
Molecular Weight:
106.14
Beilstein:
506218
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.481 (lit.)
bp
102 °C/16 mmHg (lit.)
203-208 °C (lit.)
mp
10-14 °C (lit.)
density
1.196 g/mL at 25 °C (lit.)
SMILES string
CC(S)C(O)=O
InChI
1S/C3H6O2S/c1-2(6)3(4)5/h2,6H,1H3,(H,4,5)
InChI key
PMNLUUOXGOOLSP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Thiolactic acid (TLA) can be used as a building block in the synthesis of:
It can also be used as a bidental chelating agent for the surface modification of titanium dioxide (TiO2) nanoparticles for the removal of cadmium from waste water.
- Thiolactomycin via oxathiolanone intermediate.
- 4-Thiazolidinones by reacting various Schiff bases with thioglycolic acid.
- 1,4-Naphthoquinone derivatives containing sulfur atom for antibacterial and antiviral activity studies.
It can also be used as a bidental chelating agent for the surface modification of titanium dioxide (TiO2) nanoparticles for the removal of cadmium from waste water.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of novel heterocyclic 4-thiazolidinone derivatives and their antibacterial activity
Mistry KM and Desai KR
Journal of Chemistry, 1(4), 189-193 (2004)
A Flexible Route to (5 R)-Thiolactomycin, a Naturally Occurring Inhibitor of Fatty Acid Synthesis
McFadden JM, et al.
Organic Letters, 4(22), 3859-3862 (2002)
Cadmium removal from water using thiolactic acid-modified titanium dioxide nanoparticles
Skubal LR, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 148(1-3), 393-397 (2002)
Synthesis and biological evaluation of novel 1, 4-naphthoquinone derivatives as antibacterial and antiviral agents
Tandon VK, et al.
Bioorganic & Medicinal Chemistry Letters, 15(14), 3463-3466 (2005)
Eun-Jee Oh et al.
Journal of microbiological methods, 54(3), 411-418 (2003-07-05)
To identify the metallo-beta-lactamases (MBLs) prevalent in Korea, a total of 130 clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii (99 P. aeruginosa and 31 A. baumannii) with a reduced susceptibility to imipenem (IPM) and/or ceftazidime (CAZ) was subjected to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service