All Photos(2)
About This Item
Linear Formula:
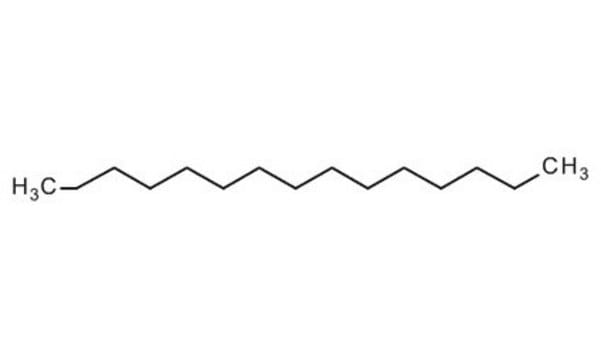
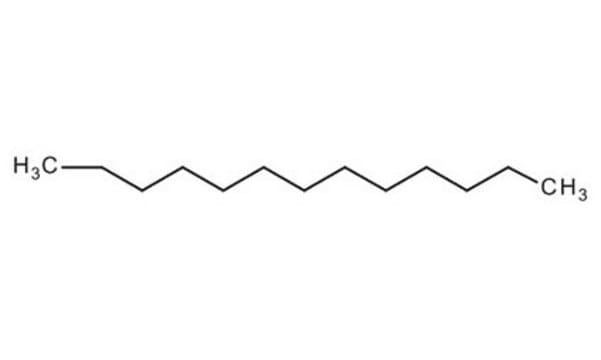
CH3(CH2)13CH3
CAS Number:
Molecular Weight:
212.41
Beilstein:
1698194
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
7.4 (vs air)
Quality Level
vapor pressure
1 mmHg ( 91.6 °C)
Assay
≥99%
expl. lim.
6.5 %
refractive index
n20/D 1.431 (lit.)
bp
270 °C (lit.)
mp
8-10 °C (lit.)
density
0.769 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCCCC
InChI
1S/C15H32/c1-3-5-7-9-11-13-15-14-12-10-8-6-4-2/h3-15H2,1-2H3
InChI key
YCOZIPAWZNQLMR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Pentadecane is a linear alkane with high latent heat (~168 kJ/kg) and low melting point. These properties make it an ideal candidate as a phase change material (PCM) for cooling applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1
Supplementary Hazards
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermal properties of n-pentadecane, n-heptadecane and n-nonadecane in the solid/liquid phase change region.
Velez C, et al.
International Journal of Thermal Sciences, 94, 139-146 (2015)
The thermodynamic properties of n-pentadecane in the liquid state, determined by the results of measurements of sound velocity.
Dovnar D, et al.
High Temperature, 39(6), 835-839 (2001)
Heber J Chacon-Madrid et al.
Environmental science & technology, 46(20), 11179-11186 (2012-09-14)
We use a two-dimensional volatility basis set (2D-VBS) box model to simulate secondary organic aerosol (SOA) mass yields of linear oxygenated molecules: n-tridecanal, 2- and 7-tridecanone, 2- and 7-tridecanol, and n-pentadecane. A hybrid model with explicit, a priori treatment of
Kevin M Foote et al.
Organic & biomolecular chemistry, 1(22), 3917-3948 (2003-12-11)
A concise synthesis of the tricyclic furanochroman unit 3 found in the PAF antagonist phomactin A (1) isolated from the marine fungus Phoma sp., is described. In complementary studies, a variety of synthetic routes towards the bicyclo[9.3.1]pentadecane ring system 4
Thembelani E Nomkoko et al.
Dalton transactions (Cambridge, England : 2003), (33)(33), 4029-4038 (2006-10-10)
The equilibrium constants of Cu(II), Zn(II), Ca(II) and Gd(III) with 1,15-bis(N,N-dimethyl)-5,11-dioxo-8-(N-benzyl)-1,4,8,12,15-pentaazapentadecane (La) have been studied at 25 degrees C and an ionic strength of 0.15 mol dm-3. Copper forms more stable complexes with La than the other metal ions investigated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service