M26305
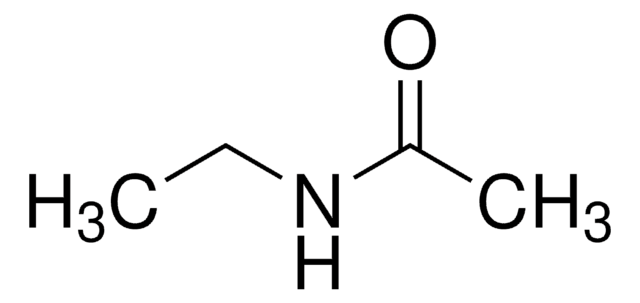
N-Methylacetamide
≥99%
Synonym(s):
Acetylmethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONHCH3
CAS Number:
Molecular Weight:
73.09
Beilstein:
1071255
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
refractive index
n20/D 1.433 (lit.)
bp
204-206 °C (lit.)
mp
26-28 °C (lit.)
density
0.957 g/mL at 25 °C (lit.)
SMILES string
CNC(C)=O
InChI
1S/C3H7NO/c1-3(5)4-2/h1-2H3,(H,4,5)
InChI key
OHLUUHNLEMFGTQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Methylacetamide can be used:
- To synthesize N-methyl-N-(3-thienyl)acetamide by reacting with 3-bromothiophene in the presence of CuI catalyst and N,N′-dimethylethylenediamine.(1)
- As a ligand to synthesize the zirconium(IV) complex, Zr(MeC(O)NMe)4 by reacting with tetrakis(dimethylamido)zirconium.(2)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
240.8 °F
Flash Point(C)
116 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hangfei Chen et al.
Environmental science & technology, 51(20), 11710-11717 (2017-09-15)
Amides represent an important class of nitrogen-containing compounds in the atmosphere that can in theory interact with atmospheric acidic particles and contribute to secondary aerosol formation. In this study, uptake coefficients (γ) of six alkylamides (C
Haibo Yu et al.
Journal of the American Chemical Society, 132(31), 10847-10856 (2010-08-05)
Most current biomolecular simulations are based on potential energy functions that treat the electrostatic energy as a sum of pairwise Coulombic interactions between effective fixed atomic charges. This approximation, in which many-body induced polarization effects are included in an average
Hochan Lee et al.
The journal of physical chemistry. A, 116(1), 347-357 (2011-11-18)
IR probes have been extensively used to monitor local electrostatic and solvation dynamics. Particularly, their vibrational frequencies are highly sensitive to local solvent electric field around an IR probe. Here, we show that the experimentally measured vibrational frequency shifts can
Jakub Kaminský et al.
The journal of physical chemistry. A, 115(1), 30-34 (2010-12-15)
For spectroscopic studies of peptide and protein thermal denaturation it is important to single out the contribution of the solvent to the spectral changes from those originated in the molecular structure. To obtain insights into the origin and size of
Bruno A C Horta et al.
Journal of computational chemistry, 33(24), 1907-1917 (2012-06-01)
Considering N-methylacetamide (NMA) as a model compound, new interaction parameters are developed for the amide function in the GROMOS force field that are compatible with the recently derived 53A6(OXY) parameter set for oxygen-containing chemical functions. The resulting set, referred to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service