D161292
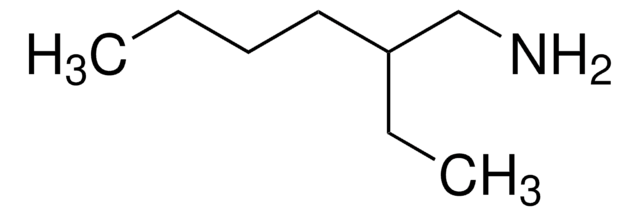
2-Amino-6-methylheptane
99%
Synonym(s):
1,5-Dimethylhexylamine, 6-Methyl-2-heptylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CH(CH2)3CH(CH3)NH2
CAS Number:
Molecular Weight:
129.24
Beilstein:
1209250
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.422 (lit.)
bp
154-156 °C (lit.)
density
0.767 g/mL at 25 °C (lit.)
SMILES string
CC(C)CCCC(C)N
InChI
1S/C8H19N/c1-7(2)5-4-6-8(3)9/h7-8H,4-6,9H2,1-3H3
InChI key
QNIVIMYXGGFTAK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Amino-6-methylheptane can be used as:
- A building block for the synthesis of n-type photoconductor, N,N′-bis(1,5-dimethylhexyl)-3,4:9,10-perylenebis (dicarbox-imide) (PDHEP).
- A reagent in the synthesis of substituted pyrrolidones by reductive amination of levulinic acid using supported platinum catalyst.
- A nucleophilic amine in the synthesis of unsymmetrical sulfamide by reacting with sulfamic acid salts and triphenylphosphine ditriflate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted synthesis of phthalocyanine-porphyrin complex and its photoelectric conversion properties
Liu MO and Hu AT
Journal of Organometallic Chemistry, 689(15), 2450-2455 (2004)
Heterogeneous Pt catalysts for reductive amination of levulinic acid to pyrrolidones
Touchy AS, et al.
ACS Catalysis, 4(9), 3045-3050 (2014)
Efficient synthesis of unsymmetrical sulfamides from sulfamic acid salts by activation with triphenylphosphine ditriflate
Shehata MF, et al.
Tetrahedron, 75(24), 3186-3194 (2019)
Pieter A Cohen et al.
Clinical toxicology (Philadelphia, Pa.), 56(6), 421-426 (2017-11-09)
The United States Food and Drug Administration banned the stimulant 1,3-dimethylamylamine (1,3-DMAA) from dietary supplements and warned consumers that the stimulant can pose cardiovascular risks ranging from high blood pressure to heart attacks. We designed our study to determine if
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service