All Photos(1)
About This Item
Empirical Formula (Hill Notation):
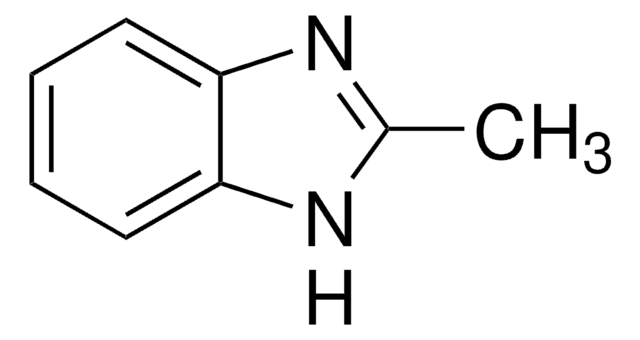
C9H10N2
CAS Number:
Molecular Weight:
146.19
Beilstein:
116595
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
mp
202-205 °C (lit.)
SMILES string
Cc1cc2nc[nH]c2cc1C
InChI
1S/C9H10N2/c1-6-3-8-9(4-7(6)2)11-5-10-8/h3-5H,1-2H3,(H,10,11)
InChI key
LJUQGASMPRMWIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Peter J Anderson et al.
Journal of bacteriology, 190(4), 1160-1171 (2007-11-06)
Corrinoid (vitamin B12-like) cofactors contain various alpha-axial ligands, including 5,6-dimethylbenzimidazole (DMB) or adenine. The bacterium Salmonella enterica produces the corrin ring only under anaerobic conditions, but it can form "complete" corrinoids aerobically by importing an "incomplete" corrinoid, such as cobinamide
Xiao-Lei Wang et al.
Journal of the American Chemical Society, 133(11), 4079-4091 (2011-02-25)
BluB is a distinct flavin destructase that catalyzes a complex oxygen-dependent conversion of reduced flavin mononucleotide (FMNH(2)) to form 5,6-dimethylbenzimidazole (DMB), the lower ligand of vitamin B(12). The catalyzed mechanism remains a challenge due to the discrepancy between the complexity
Ruibing Wang et al.
Dalton transactions (Cambridge, England : 2003), (18)(18), 3584-3589 (2009-04-22)
Cucurbit[7]uril (CB[7]) forms very stable complexes with the alpha-axial 5,6-dimethylbenzimidazole (alpha-DMB) nucleotide base when dissociated from the Co(III) center in vitamin B(12) (CNCbl, K(CB[7]) = (7.5 +/- 0.5) x 10(4) dm(3) mol(-1)) and coenzyme B(12) (AdoCbl, K(CB[7]) = (3.02 +/-
V Krishnakumar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 68(3), 811-816 (2007-05-04)
The vibrational spectra of 5,6-dimethyl benzimidazole (5,6DBZ) have been computed using the standard B3LYP/6-311G** method and basis set combinations. The solid phase FT-IR and FT-Raman spectra were recorded in the region 4000-400 and 3500-100 cm(-1), respectively. A close agreement was
Biochemistry: molecular cannibalism.
Steven E Ealick et al.
Nature, 446(7134), 387-388 (2007-03-23)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service