All Photos(1)
About This Item
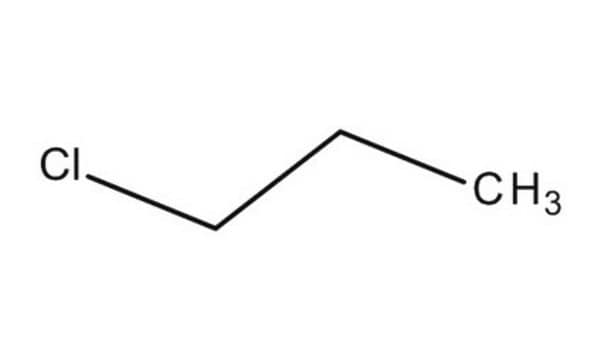
Linear Formula:
CH3CH2CH2Cl
CAS Number:
Molecular Weight:
78.54
Beilstein:
1730771
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.71 (vs air)
Quality Level
vapor pressure
5.51 psi ( 20 °C)
Assay
98%
form
liquid
autoignition temp.
968 °F
expl. lim.
11 %
refractive index
n20/D 1.388 (lit.)
bp
46-47 °C (lit.)
mp
−123 °C (lit.)
density
0.892 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCCCl
InChI
1S/C3H7Cl/c1-2-3-4/h2-3H2,1H3
InChI key
SNMVRZFUUCLYTO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Theoretical and experimental studies of the kinetics of the reaction of 1-chloropropane and fully deuterated 1-chloropropane with atomic chlorine: This study explores the reaction kinetics of 1-chloropropane with atomic chlorine, providing insights into the influence of carbon position in molecular interactions (L Fojcik et al., 2021).
- High-pressure phase equilibrium in the {carbon dioxide (1)+ 1-chloropropane (2)} binary system: Research determining the phase equilibrium properties of 1-chloropropane under various conditions, useful for industrial applications involving carbon dioxide and 1-chloropropane (M Chorazewski et al., 2015).
- Temperature dependent dielectric relaxation studies of halopropane from 10 Mhz to 50 Ghz using a time domain reflectometry (TDR): This study investigates the dielectric properties of 1-chloropropane across a broad frequency range, highlighting its applications in the pharmaceutical and pesticide industries (RV Shinde et al., 2020).
- A Study on Subchronic Inhalation Toxicity of 1-Chloropropane: An examination of the inhalation toxicity of 1-chloropropane, providing critical data for occupational safety and health assessments (Y Hyun Chung et al., 2015).
- Thermal decomposition of 1-chloropropane behind the reflected shock waves in the temperature range of 1015–1220 K: A comprehensive analysis of the decomposition mechanisms of 1-chloropropane under high-temperature conditions, significant for understanding thermal stability and reaction kinetics (G Sudhakar et al., 2014).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marta Babicka et al.
Molecules (Basel, Switzerland), 25(7) (2020-04-02)
Cellulose nanocrystals were prepared using ionic liquids (ILs), 1-ethyl-3-methylimidazolium chloride [EMIM][Cl] and 1-propyl-3-methylimidazolium chloride [PMIM][Cl], from microcrystalline cellulose. The resultant samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), scanning electron microscopy (SEM)
Jianan Gao et al.
Environmental monitoring and assessment, 192(2), 143-143 (2020-01-29)
Quenching agents (QAs) are widely used in order to prevent the additional formation of disinfection by-products (DBPs) during the sample holding time. In addition, DBP levels are usually stabilized by adjusting the pH of water samples. Previous studies have mostly
Paulina Laszuk et al.
Molecules (Basel, Switzerland), 25(16) (2020-08-19)
In this examination, we investigated the effect of lipoic acid (LA) on the properties of biological membrane models (monolayers, bilayers, and liposomes) formed from phosphatidylcholine (PC) or phosphatidylserine (PS) using the Langmuir, microelectrophoresis, and interfacial tension methods. The Langmuir technique
Marzie Sadat Mirhosseyni et al.
International journal of biological macromolecules, 175, 432-442 (2021-02-08)
Iron and nitrogen-doped carbon substances with abundant active sites that related to dispersion of heteroatom species (Fe and N) on the surface of carbonous structure, are promising choice for eco-friendly catalytic reactions. Herein, cellulose-based ionic liquid (IL) derivative not only
Rashmi Ramachandra et al.
Glycobiology, 27(5), 438-449 (2017-01-29)
Glycosaminoglycans (GAGs), such as chondroitin sulfate (CS) and dermatan sulfate (DS) from various vertebrate and invertebrate sources are known to be involved in diverse cellular mechanisms during repair and regenerative processes. Recently, we have identified CS/DS as the major GAG
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service