90303
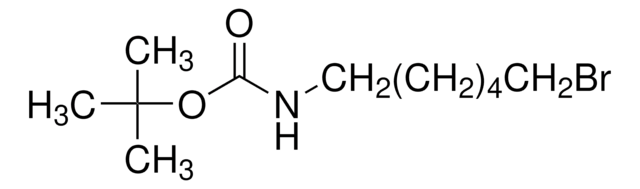
4-(Boc-amino)butyl bromide
technical, ≥90% (AT)
Synonym(s):
Carbamic acid, (4-bromobutyl)-, 1,1-dimethylethyl ester, tert-Butyl N-(4-bromobutyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)4NHCO2C(CH3)3
CAS Number:
Molecular Weight:
252.15
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
Assay
≥90% (AT)
form
liquid
reaction suitability
reagent type: cross-linking reagent
impurities
~5% 1-boc-pyrrolidine
functional group
Boc
amine
bromo
storage temp.
2-8°C
SMILES string
BrCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H18BrNO2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7H2,1-3H3,(H,11,12)
InChI key
GKGFAEREWWZBKY-UHFFFAOYSA-N
Related Categories
Application
4-(Boc-amino)butyl bromide can be used:
- For the synthesis of N-Boc-aminoalkoxyphenyl derivatives, precursor to pharmacophore elements for the treatment of glaucoma.
- For the synthesis of various aloperine derivatives with potential application as anti-HIV agents.
- For the modification of 4,5,6,7-tetrabromobenzotriazole (TBB) derivatives to generate improved CK2 inhibitors.
Other Notes
Building block for the synthesis of natural products and biologically active compounds.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Identification of bivalent ligands with melatonin receptor agonist and fatty acid amide hydrolase (FAAH) inhibitory activity that exhibit ocular hypotensive effect in the rabbit.
Spadoni G, et al.
Journal of medicinal chemistry, 61(17), 7902-7916 (2018)
Synthesis, biological activity and structural study of new benzotriazole-based protein kinase CK2 inhibitors.
Swider R, et al.
Royal Society of Chemistry Advances, 5(89), 72482-72494 (2015)
Structure Optimization of Aloperine Derivatives as HIV-1 Entry Inhibitors.
Dang Z, et al.
ACS Medicinal Chemistry Letters, 8(11), 1199-1203 (2017)
D.L. Selwood et al.
Journal of Medicinal Chemistry, 4, 78-78 (2001)
H. Ina et al.
The Journal of Organic Chemistry, 61, 1023 -1023 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service