About This Item
Recommended Products
Quality Level
Assay
95%
form
powder
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
SMILES string
CC1=CC=CC=C1[Ni]Cl.CN(C)CCN(C)C
InChI
1S/C7H7.C6H16N2.ClH.Ni/c1-7-5-3-2-4-6-7;1-7(2)5-6-8(3)4;;/h2-5H,1H3;5-6H2,1-4H3;1H;/q;;;+1/p-1
InChI key
NMLMESVZRUMFAE-UHFFFAOYSA-M
Related Categories
Application
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Palladium catalysts in transition metal-catalyzed coupling reactions offer diversity, accessibility, and robustness over nickel catalysts.
Related Content
Research in the Doyle group focuses on two areas: nucleophilic fluorination and nickel catalysis. The Doyle group has developed several reagents that advance these research areas. In fluorination, 2-pyridinesulfonyl fluoride (PyFluor) can be used for the mild deoxyfluorination of primary and secondary alcohols, a procedure which is normally accomplished by the sensitive reagent DAST. In nickel catalysis, the Doyle group has developed a modular, air-stable nickel precatalyst, [(TMEDA)Ni(o-tolyl)Cl], which has broad utility for a wide variety of reactions. This precatalyst can be used in place of Ni(cod)2, NiCl2, as well as other reported precatalysts. Doyle has also reported electron-deficient olefin ligands as a new class of ligand for accelerated reductive elimination. In particular, the sultam-derived ligand Fro-DO has been found to be critical for high yields in the cross-coupling of tertiary aziridines to form quaternary centers.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service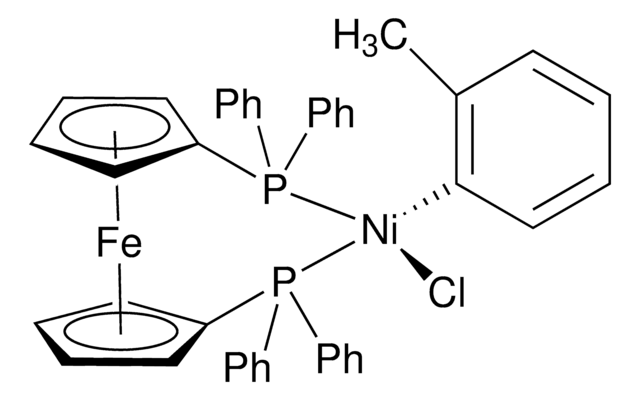

![[(TEEDA)Ni(o-tolyl)Cl] ≥95%](/deepweb/assets/sigmaaldrich/product/structures/156/227/a6ce708d-c671-4ca6-98ba-ef780504ca58/640/a6ce708d-c671-4ca6-98ba-ef780504ca58.png)


![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)

![[1,2-Bis(diphenylphosphino)ethane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/707/956/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf/640/483e7a6e-5fb5-4e39-abd1-ecf33ccab3cf.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)