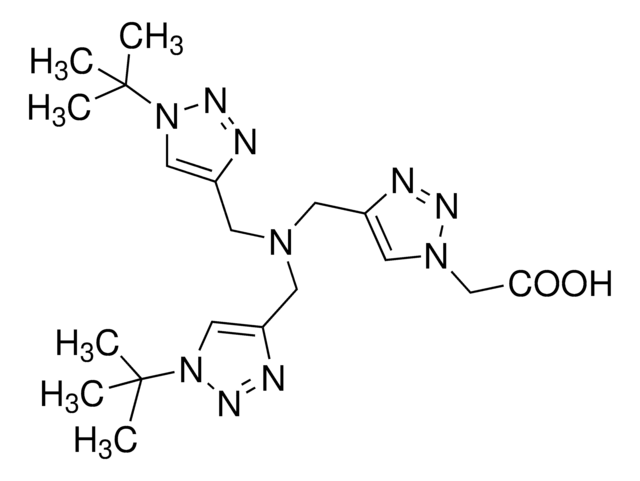
760757
Azide-fluor 545
Synonym(s):
5-Carboxytetramethylrhodamine-azide, TAMRA PEG azide., TAMRA-azide, Tetramethylrhodamine
About This Item
Recommended Products
Related Categories
Application
Spectral Properties: Abs/Em = 546/565 nm
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)