73861
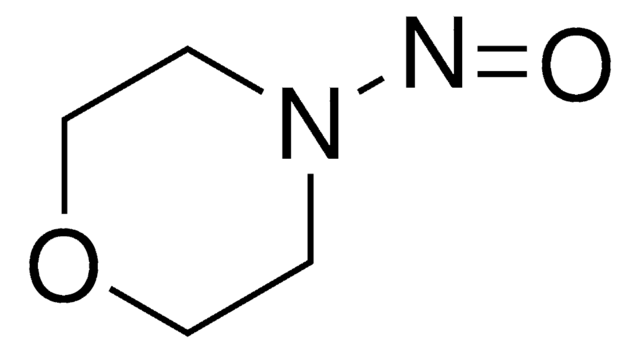
N-Nitrosodiethylamine
≥99.0% (GC)
Synonym(s):
Diethylnitrosamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)2NNO
CAS Number:
Molecular Weight:
102.14
Beilstein:
1744991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (GC)
form
liquid
IVD
for in vitro diagnostic use
bp
177 °C (lit.)
density
0.95 g/mL (lit.)
functional group
N-nitroso
amine
nitroso
SMILES string
CCN(CC)N=O
InChI
1S/C4H10N2O/c1-3-6(4-2)5-7/h3-4H2,1-2H3
InChI key
WBNQDOYYEUMPFS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Nitrosodiethylamine can be used as a reactant to synthesize:
- α-Amino oxime derivatives from alkenes via visible-light-induced photoaddition in the presence of HCl.
- Diethylnitrosamine metalloporphyrin complexes from metalloporphyrins in the presence of dichloromethane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Chronic 3 - Carc. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dilip V Patil et al.
Organic letters, 23(8), 3105-3109 (2021-04-02)
The generation of aminium radical cation species from N-nitrosoamines is disclosed for the first time through visible-light excitation at 453 nm. The developed visible-light-promoted photoaddition reaction of N-nitrosoamines to alkenes was combined with the o-NQ-catalyzed aerobic oxidation protocol of amines
Changyu Zhu et al.
Journal of hepatology, 74(3), 613-626 (2020-10-11)
The hepatocyte Notch pathway is a pathogenic factor in non-alcoholic steatohepatitis (NASH)-associated fibrosis, but its role in hepatocellular carcinoma (HCC) is less well defined. Herein, we aimed to characterize the molecular and clinical features of Notch-active human HCC, and to
Li Chen et al.
Inorganic chemistry, 37(18), 4677-4688 (2001-10-24)
Diethylnitrosamine reacts with [(TPP)Fe(THF)(2)]ClO(4) (TPP = 5,10,15,20-tetraphenylporphyrinato dianion) in toluene to generate the bis-nitrosamine complex, [(TPP)Fe(Et(2)NNO)(2)]ClO(4), in 96% isolated yield. The related [(TTP)Fe(Et(2)NNO)(2)]SbF(6) (TTP = 5,10,15,20-tetra-p-tolylporphyrinato dianion) complex is prepared in 70% isolated yield via a similar reaction in CH(2)Cl(2).
Koichi Fujisawa et al.
PloS one, 10(6), e0129950-e0129950 (2015-06-25)
Maid is a helix-loop-helix protein that is involved in cell proliferation. In order to further elucidate its physiological functions, we studied Maid activity in two small fish model systems. We found that Maid expression was greatest in zebrafish liver and
Bo Zhang et al.
Theranostics, 11(10), 4743-4758 (2021-03-24)
Aims: Emerging evidence is demonstrating that rapid regeneration of remnant liver elicited by associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) may be attenuated in fibrotic livers. However, the molecular mechanisms responsible for this process are largely
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service