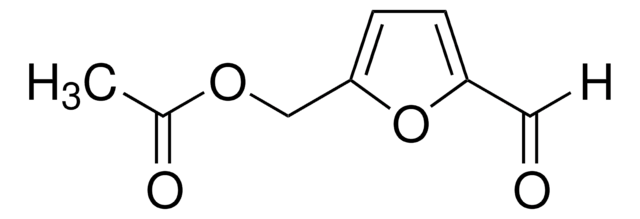
728373
2,5-Furandicarboxaldehyde
97%
Synonym(s):
2,5-Diformylfuran, 2,5-Furandicarbaldehyde, 5-Formylfurfural
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H4O3
CAS Number:
Molecular Weight:
124.09
Beilstein:
109424
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥96.5% (HPLC)
97%
form
powder
storage temp.
−20°C
SMILES string
O=Cc1ccc(C=O)o1
InChI
1S/C6H4O3/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChI key
PXJJKVNIMAZHCB-UHFFFAOYSA-N
General description
2,5-Furandicarboxaldehyde is an oxidation product of 5-hydroxymethyl furfural. It is used as an organic building block in chemical synthesis. It is also used as a precursor for the production of valuable biopolymers.
Application
2,5- Furandicarboxaldehyde can be used in the synthesis of sustainable thin–film composite (TFC) membranes by interfacial polymerization reaction with chitosan and it also acts as a fluorescent chemo sensor for Hg2+ ions.
2,5-Furandicarboxaldehyde can be used as a building block in the fabrication of sustainable thin-film composite (TFC) membranes by the interfacial polymerization reaction with chitosan.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solvent-resistant thin-film composite membranes from biomass-derived building blocks: chitosan and 2, 5-furandicarboxaldehyde
Park, et al.
ACS sustainable chemistry & engineering, 10, 998-1007 (2021)
A bis-hydrazone derivative of 2, 5-furandicarboxaldehyde with perfect hetero-atomic cavity for selective sensing of Hg (II) and its intracellular detection in living HeLa S3 cell
Kumari, et al.
Sensors and Actuators B, Chemical, 243, 1181-1190 (2017)
Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2, 5-furandicarboxyaldehyde in aqueous suspension of g-C3N4
Krivtsov, et al.
Applied Catalysis. B, Environmental, 204, 430-439 (2017)
Cristina Megías-Sayago et al.
Frontiers in chemistry, 8, 461-461 (2020-06-26)
A series of gold catalysts supported on pure CeO2, ZrO2, and two different Ce-Zr mixed oxides have been prepared and tested in the 5-hydroxymethyl-2-furfural oxidation reaction. All catalysts show high catalytic activity (100% conversion) and important selectivity (27-41%) to the
Maria Ventura et al.
ChemSusChem, 11(8), 1305-1315 (2018-03-08)
Mixed oxides based on MgO⋅CeO2 were used as efficient catalysts in the aerobic oxidation of 5-hydroxymethylfurfural (5-HMF) to afford, with very high selectivity, either 2,5-diformylfuran (DFF, 99 %) or 2-formyl-5-furancarboxylic acid (FFCA, 90 %), depending on the reaction conditions. 5-Hydroxymethyl-2-furancarboxylic acid (HMFCA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)