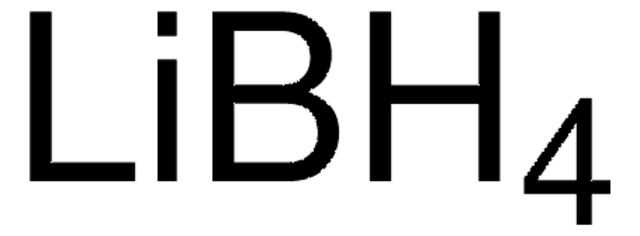702714
Lithium borohydride solution
0.5 M in diethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
LiBH4
CAS Number:
Molecular Weight:
21.78
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
0.5 M in diethyl ether
density
0.719 g/mL at 25 °C
SMILES string
[Li+].[H][B-]([H])([H])[H]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Lithium borohydride is a commonly used reducing agent. It can also be used a reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes.
- Mechano-chemical metathesis reactions.
- Noncatalytic hydrolysis for hydrogen generation.
- Growth of large gold monolayer protected-clusters.
- Anion substitution reactions.
- Dehydrogenation reactions.
Reactant for:
- Preparation of gallium, indium, rhenium and zinc tris(mercaptoimidazolyl)hydroborato complexes
- Mechano-chemical metathesis reactions
- Noncatalytic hydrolysis for hydrogen generation
- Growth of large gold monolayer protected-clusters
- Anion substitution reactions
- Dehydrogenation reactions
Other Notes
Material may form precipitate on standing, which does not affect its use.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
Flash Point(F)
-40.0 °F
Flash Point(C)
-40 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mechano-chemical reactions in LiBH4+ VCln (n= 2 and 3) mixtures.
Llamas-Jansa I, et al.
J. Alloy Compounds, 509, S684-S687 (2011)
Hydrogen generation from noncatalytic hydrolysis of LiBH4/NH3BH3 mixture for fuel cell applications.
Weng B, et al.
International Journal of Hydrogen Energy, 36(17), 10870-10876 (2011)
Controlled growth and catalytic activity of gold monolayer protected clusters in presence of borohydride salts.
Dasog M, et al.
Chemical Communications (Cambridge, England), 47(30), 8569-8571 (2011)
Effect of hydrogen back pressure on dehydrogenation behavior of LiBH4-based reactive hydride composites.
Shim J H, et al.
The Journal of Physical Chemistry Letters, 1(1), 59-63 (2009)
Synthesis, crystal structure, and thermal properties of the first mixed-metal and anion-substituted rare earth borohydride LiCe (BH4) 3Cl.
Frommen c, et al.
The Journal of Physical Chemistry, 115(47), 23591-23602 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service