689092
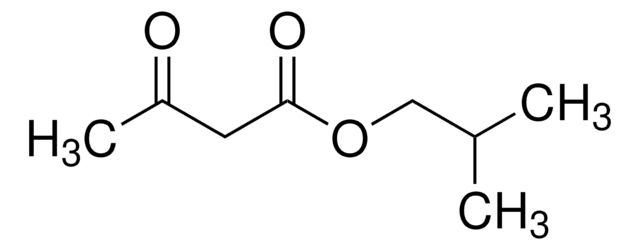
Isopropyl acetoacetate
Arxada quality, ≥99.0% (GC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCH2COOCH(CH3)2
CAS Number:
Molecular Weight:
144.17
Beilstein:
1757046
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (GC)
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
impurities
≤0.10% acid (as acetic acid)
≤0.10% water
≤0.5% ethyl acetoacetate
bp
95 °C/52 hPa (lit.)
density
0.989 g/mL at 20 °C (lit.)
SMILES string
CC(C)OC(=O)CC(C)=O
InChI
1S/C7H12O3/c1-5(2)10-7(9)4-6(3)8/h5H,4H2,1-3H3
InChI key
GVIIRWAJDFKJMJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Isopropyl acetoacetate can be used as a reactant to synthesize:
- Isopropyl 2-acetyl-3-phenyl-2-propenoate via Knoevenagel reaction in the presence of Ti(O-i-Pr)4.
- Substituted 1,4-dihydropyridines by reacting with cinnamaldehydes and primary amines.
- 5-Isopropoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-thione via Biginelli reaction with benzaldehyde and thiourea.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Organocatalytic asymmetric three-component cyclization of cinnamaldehydes and primary amines with 1, 3-dicarbonyl compounds: straightforward access to enantiomerically enriched dihydropyridines
Jiang J, et al.
Angewandte Chemie (International Edition in English), 47(13), 2458-2462 (2008)
Novel Knoevenagel-type reaction via titanium enolate derived from Ti(Oi-Pr)4 and diketene
Hayashi M, et al.
Tetrahedron, 60(32), 6777-6783 (2004)
Synthesis and biological evaluation of novel isopropyl 2-thiazolopyrimidine-6-carboxylate derivatives
Kotaiah Y, et al.
J. Korean Chem. Soc., 56(1), 68-73 (2012)
M M V Ramana et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 152, 165-171 (2015-07-25)
In the present work, isopropyl-6-amino-4-(3,5-bis(trifluoromethyl)phenyl)-5-cyano-2-methyl-4H-pyran-3-carboxylate (4H-pyran analog) has been synthesized by a three component reaction catalyzed by CsOH/γ-Al2O3 and characterized. The interaction of 4H-pyran analog with herring sperm DNA (hs DNA) under physiological conditions (phosphate buffer of pH 7.2) was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service