All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H11NO
CAS Number:
Molecular Weight:
149.19
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
optical activity
[α]/D -23.0°, c = 1 in ethanol
mp
142-146 °C
functional group
hydroxyl
SMILES string
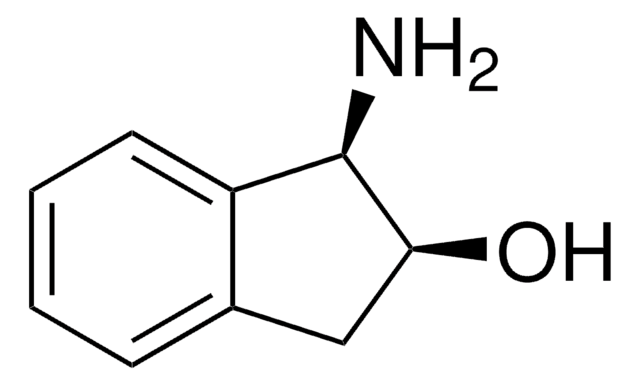
N[C@H]1[C@H](O)Cc2ccccc12
InChI
1S/C9H11NO/c10-9-7-4-2-1-3-6(7)5-8(9)11/h1-4,8-9,11H,5,10H2/t8-,9-/m1/s1
InChI key
LOPKSXMQWBYUOI-RKDXNWHRSA-N
Application
(1R,2R)-(−)-trans-1-Amino-2-indanol has been used as a starting material to prepare an indene-based chiral auxiliary, which is used in the aldol reaction.
It can also be used as:
It can also be used as:
- A starting material in the synthesis of oxazoline-alcohol ligands, which are employed in the asymmetric addition reaction of diethylzinc to aldehydes.
- A chiral test compound in the study of enantiomeric separation of chiral primary amines using supercritical fluid chromatography (SFC) and HPLC.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Indene-based thiazolidinethione chiral auxiliary for propionate and acetate aldol additions
Osorio-Lozada A and Olivo HF
Organic Letters, 10(4), 617-620 (2008)
Comparison of enantiomeric separations and screening protocols for chiral primary amines by SFC and HPLC
Armstrong DW, et al.
LCGC North America, 32(17), 742-752 (2014)
Efficient one-step synthesis of chiral bidentate oxazoline-alcohol ligands via a cyclic imidate ester rearrangement
Noel T, et al.
Tetrahedron Asymmetry, 20(17), 1962-1968 (2009)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service