633305
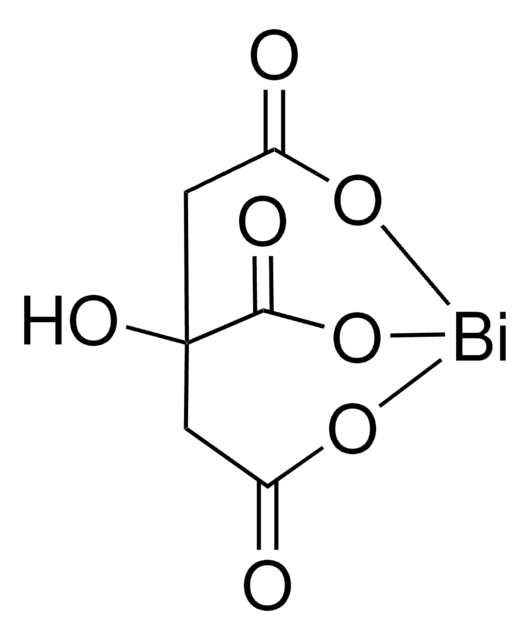
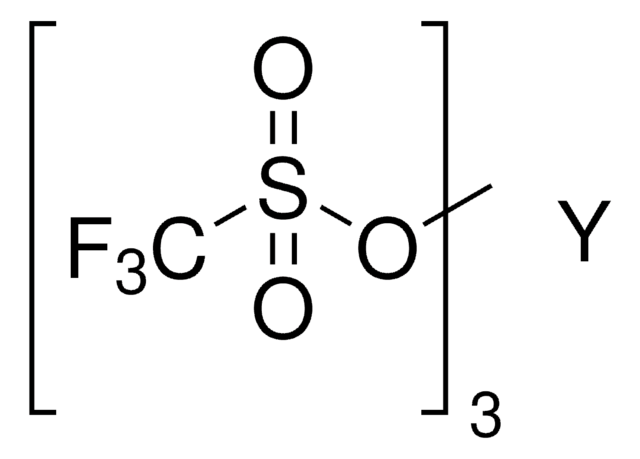
Bismuth(III) trifluoromethanesulfonate
Synonym(s):
Bi(OTf)3, Bismuth tris(trifluoromethanesulfonate), Bismuth(III) triflate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Bi(OSO2CF3)3
CAS Number:
Molecular Weight:
656.19
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
reaction suitability
core: bismuth
reagent type: catalyst
mp
>300 °C (lit.)
SMILES string
[Bi+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Bi/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
NYENCOMLZDQKNH-UHFFFAOYSA-K
Looking for similar products? Visit Product Comparison Guide
Application
Bismuth(III) trifluoromethanesulfonate may be used as a catalyst in the following processes:
- deprotection of acetals
- cleavage of 2-tert-butoxy derivatives of thiophenes and furans
- allylation of acetals to form homoallyl ethers
Catalyzes direct substitution of allylic, propargylic, and benzylic alcohols with sulfonamides, carbamates, and carboxamides.
Packaging
Bismuth(III) trifluoromethanesulfonate is an eco-friendly Lewis acid that can be prepared by reacting triflic acid with bismuth(III) trifluoroacetate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bismuth (III) triflate in organic synthesis.
Gaspard-Iloughmane H and Le Roux C.
European Journal of Organic Chemistry, 2004(12), 2517-2532 (2004)
Applications of bismuth (III) compounds in organic synthesis.
Leonard NM, et al.
Tetrahedron, 58(42), 8373-8397 (2002)
Bismuth (III) trifluoromethanesulfonate: An efficient catalyst for the sulfonylation of arenes.
Repichet S, et al.
The Journal of Organic Chemistry, 64, 6479-6482 (1999)
Bismuth-catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides.
Hongbo Qin et al.
Angewandte Chemie (International ed. in English), 46(3), 409-413 (2006-12-06)
Maria Ricciardi et al.
ChemSusChem, 10(10), 2291-2300 (2017-04-05)
The disposal of any waste by recovering it within the production plant represents the ultimate goal of every biorefinery. In this scenario, the selective preparation of monoalkyl glyceryl ethers (MAGEs) starting from glycidol, obtained as byproduct in the epichlorohydrin production
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service