568554
Tri(ethylene glycol) monoethyl ether
technical grade
Synonym(s):
Triethylene glycol monoethyl ether, Ethyltriglycol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
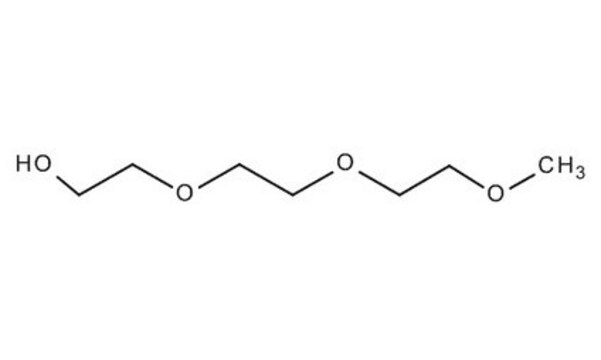
Linear Formula:
CH3CH2(OCH2CH2)3OH
CAS Number:
Molecular Weight:
178.23
Beilstein:
1700466
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
Quality Level
refractive index
n20/D 1.438 (lit.)
bp
256 °C (lit.)
density
1.020 g/mL at 25 °C (lit.)
SMILES string
CCOCCOCCOCCO
InChI
1S/C8H18O4/c1-2-10-5-6-12-8-7-11-4-3-9/h9H,2-8H2,1H3
InChI key
WFSMVVDJSNMRAR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
269.6 °F - closed cup
Flash Point(C)
132 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marco Lucarini et al.
Journal of the American Chemical Society, 126(30), 9326-9329 (2004-07-30)
EPR spectroscopy has been used to study the interaction of para-substituted benzyl hydroxyalkyl nitroxides with the monolayer of water-soluble protected gold cluster made by a short alkyl chain and a triethylene glycol monomethyl ether unit. The inclusion of nitroxide probes
Isabella Orienti et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 54(2), 229-233 (2002-08-23)
Among the different methods used to increase the aqueous drug solubility, the preparation of a solid dispersion with a soluble carrier represents an interesting formulative approach. We substituted polyvinylalcohol with triethyleneglycolmonoethylether and obtained a suitable material for the formulation of
I Orienti et al.
Biomacromolecules, 6(5), 2875-2880 (2005-09-13)
A series of poly(vinyl alcohol) amphiphilic derivatives have been prepared to obtain polymeric aggregates in aqueous phase holding thermodynamic instability. The aim was to evaluate their ability to interact with tumor cells eliciting selective cytotoxicity. The poly(vinyl alcohol) derivatives were
[Establishment of the maximum permissible concentration triethylene glycol ethyl ether in reservoir water].
V A Kondratiuk et al.
Gigiena i sanitariia, (5)(5), 84-85 (1982-05-01)
José F Salmerón et al.
Sensors (Basel, Switzerland), 18(7) (2018-07-18)
This work describes a fully wireless sensory system where a chipless strategy is followed in the sensor part. Alternatively, to characterize only the sensing element, we present the response of the reader antenna when the sensing element is placed in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service