All Photos(1)
About This Item
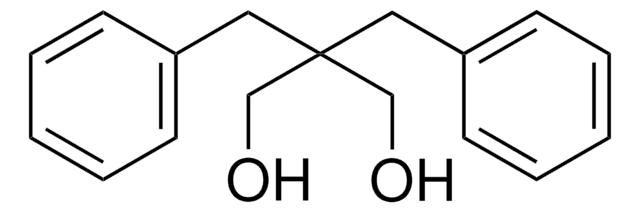
Linear Formula:
HOCH2CH(C6H5)CH2OH
CAS Number:
Molecular Weight:
152.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
53-56 °C (lit.)
functional group
hydroxyl
phenyl
SMILES string
OCC(CO)c1ccccc1
InChI
1S/C9H12O2/c10-6-9(7-11)8-4-2-1-3-5-8/h1-5,9-11H,6-7H2
InChI key
BPBDZXFJDMJLIB-UHFFFAOYSA-N
Application
2-Phenyl-1,3-propanediol may be used to synthesize enantiomers of 2-phenyl-3-hydroxypropylcarbamate, via a chemoenzymatic method. It may also be employed in the preparation of 2-phenyl-1,3-propanedithiol.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, characterization and electrochemistry of phenyl-functionalized diiron propanedithiolate complexes.
Li CG, et al.
Polyhedron, 67, 416-421 (2014)
J Ríha et al.
Veterinarni medicina, 36(2), 65-69 (1991-02-01)
In practical farming conditions of an agricultural enterprise situated in South-East Moravia, the repellents N,N'-diethyl-m-toluamide and 2-phenyl propanediol 1,3 were tested after their application to grazing first-calves of the Bohemian Pied breed. The experiment was conducted in July and August
Ryoji Mitsui et al.
Bioscience, biotechnology, and biochemistry, 71(8), 1858-1864 (2007-08-11)
Bacillus cereus 809A and Burkholderia sp. 711C were isolated from soil. These strains demonstrate hydrolysis activity towards prochiral 2-phenyl-1,3-propanediol diacetate and accumulated the corresponding chiral monoacetates into the reaction mixture. When 2-phenyl 1,3-propanediol diacetate was used as a substrate, the
A chemoenzymatic synthesis of both enantiomers of 2-phenyl-3-hydroxypropylcarbamate, a metabolite of felbamate.
Morgan B, et al.
Tetrahedron Asymmetry, 6(7), 1765-1772 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service