All Photos(1)
About This Item
Empirical Formula (Hill Notation):
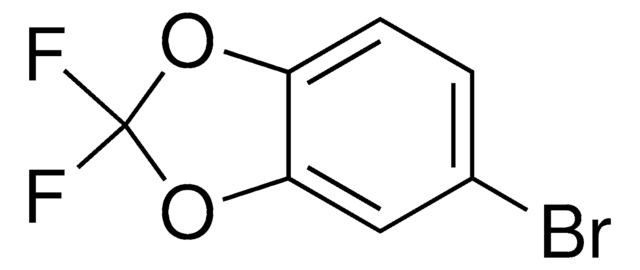
C8H7BrO2
CAS Number:
Molecular Weight:
215.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.588 (lit.)
bp
259-260 °C (lit.)
density
1.598 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Brc1ccc2OCCOc2c1
InChI
1S/C8H7BrO2/c9-6-1-2-7-8(5-6)11-4-3-10-7/h1-2,5H,3-4H2
InChI key
LFCURAJBHDNUNG-UHFFFAOYSA-N
General description
6-Bromo-1,4-benzodioxane is an aryl halide. It can be synthesized from 1,4-benzodioxane, via bromination with bromine in acetic acid. It undergoes alkoxycarbonylation reaction with sodium tert-butoxide in the presence of CO to yield 2,3-dihydro-benzo[1,4]dioxine-6-carboxylic acid t-butyl ester.
Application
6-Bromo-1,4-benzodioxane may be used as a starting reagent in the synthesis of chiral diphosphines (SYNPHOS and DIFLUORPHOS).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chiral biphenyl diphosphines for asymmetric catalysis: Stereoelectronic design and industrial perspectives.
Jeulin, Severine, et al.
Proceedings of the National Academy of Sciences of the USA, 101(16), 5799-5804 (2004)
An Efficient Method for the Preparation of Tertiary Esters by Palladium-Catalyzed Alkoxycarbonylation of Aryl Bromides.
Xin, Zhuo, et al.
Organic Letters, 14(1), 284-287 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service