532363
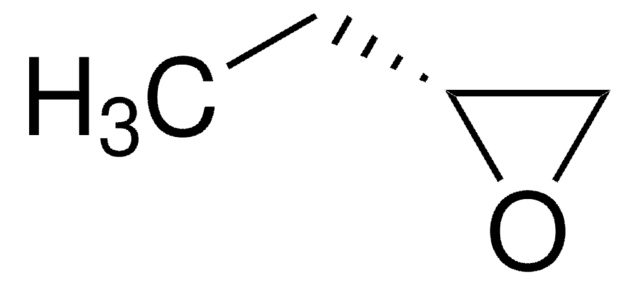
(S)-(−)-1,2-Epoxybutane
98%
Synonym(s):
(2S)-Ethyloxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O
CAS Number:
Molecular Weight:
72.11
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]20/D −10°, neat
refractive index
n20/D 1.386 (lit.)
bp
63 °C (lit.)
density
0.837 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CC[C@H]1CO1
InChI
1S/C4H8O/c1-2-4-3-5-4/h4H,2-3H2,1H3/t4-/m0/s1
InChI key
RBACIKXCRWGCBB-BYPYZUCNSA-N
Application
(S)-(−)-1,2-Epoxybutane can be used:
- As a starting material to prepare (+)- and (−)-homononactic acids, which are used as intermediates in the total synthesis of a cyclic antibiotic tetranactin.
- To prepare a chiral phosphorus synthon, which is applicable in the synthesis of phytoprostane B1 type I.
- To prepare Eu3+-based precatalysts applicable in the Mukaiyama Aldol reaction in water.
Legal Information
Manufactured under license by Sterling Pharma Solutions Limited, using Jacobsen HKR technology.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
10.0 °F - closed cup
Flash Point(C)
-12.2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A flexible synthesis of the phytoprostanes B1 type I and II
El Fangour S, et al.
The Journal of Organic Chemistry, 70(3), 989-997 (2005)
Synthesis, spectroscopic characterization, and reactivity of water-tolerant Eu3+-based precatalysts
Averill DJ and Allen MJ
Inorganic Chemistry, 53(12), 6257-6263 (2014)
Synthesis of (+)-and (−)-homononactic acid from (S)-1, 2-epoxybutane. Total synthesis of tetranactin by ′reverse coupe du roi′
Schmidt U and Werner J
Journal of the Chemical Society. Chemical Communications, 996-998 (1986)
Hong Yuan Sun et al.
Carbohydrate research, 344(15), 1999-2004 (2009-08-26)
A new soluble cyclodextrin derivative 6-O-(2-hydroxybutyl)-beta-cyclodextrin (6-HB-beta-CD) was prepared. Its molecular binding and recognition ability were investigated with the comparison of beta-cyclodextrin (beta-CD), 2-O-(2-hydroxypropyl)-beta-cyclodextrin (2-HP-beta-CD), 6-O-(2-hydroxypropyl)-beta-cyclodextrin (6-HP-beta-CD), and 2-O-(2-hydroxybutyl)-beta-cyclodextrin (2-HB-beta-CD). The relationship between the complex stability constants and the possible
M Katz et al.
Journal of environmental pathology and toxicology, 3(5-6), 171-187 (1980-06-01)
Nitrosopiperidine, sodium nitrite and 1,2 epoxybutane were tested in the Ames agar incorporation assay in an attempt to establish exact criteria for detecting the activity of these weak mutagens. As regards minimum concentrations it was determined that at 500 microgram
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service