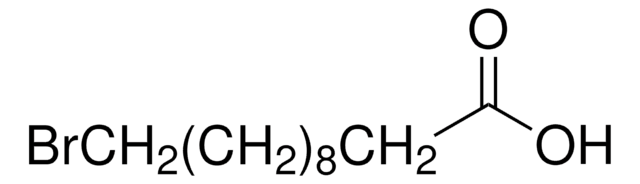All Photos(1)
About This Item
Linear Formula:
HO(CH2)10CO2H
CAS Number:
Molecular Weight:
202.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
functional group
carboxylic acid
hydroxyl
SMILES string
OCCCCCCCCCCC(O)=O
InChI
1S/C11H22O3/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h12H,1-10H2,(H,13,14)
InChI key
KNRCBASNXNXUQQ-UHFFFAOYSA-N
Application
11-Hydroxyundecanoic acid can be prepared by employing the following starting reagents:
- 10-undecenoic acid
- undecylenic acid and hydrobromic acid
- methyl 11-bromoundecanoate
- ricinoleic acid (12-hydroxyoleic acid)
11-Hydroxyundecanoic acid may be employed for the preparation of higher molecular weight polyesters.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bis-3-methyl-2-butylborane as a selective reagent for the reduction of representative functional groups.
Brown HC and Bigley DB.
Journal of the American Chemical Society, 83(2), 486-486 (1982)
Studies on ω-Oxidation of Fatty Acids in vitro.
Kamei S, et al.
Journal of Biochemistry, 56(1), 72- 76 (1964)
Preparation of Macrocyclic Lactones by Depolymerization1.
Spanagel EW and Carothers WH.
Journal of the Chemical Society, 58(4), 654-656 (1936)
Chemo-enzymatic synthesis of 11-hydroxyundecanoic acid and 1, 11-undecanedioic acid from ricinoleic acid.
Jang HY, et al.
Green Chemistry, 18(4), 1089-1095 (2016)
Enzyme-catalysed condensation polymerization of 11-hydroxyundecanoic acid with lipase from Candida cylindracea.
O'Hagan D and Zaidi NA.
Polymer, 35(14), 3576-3578 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service