All Photos(1)
About This Item
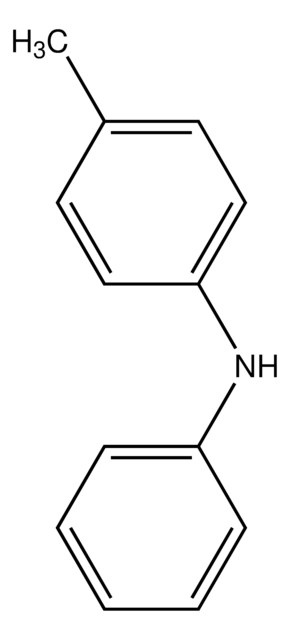
Linear Formula:
(C6H5)2NCH3
CAS Number:
Molecular Weight:
183.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.623 (lit.)
bp
293 °C (lit.)
density
1.05 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(c1ccccc1)c2ccccc2
InChI
1S/C13H13N/c1-14(12-8-4-2-5-9-12)13-10-6-3-7-11-13/h2-11H,1H3
InChI key
DYFFAVRFJWYYQO-UHFFFAOYSA-N
Related Categories
General description
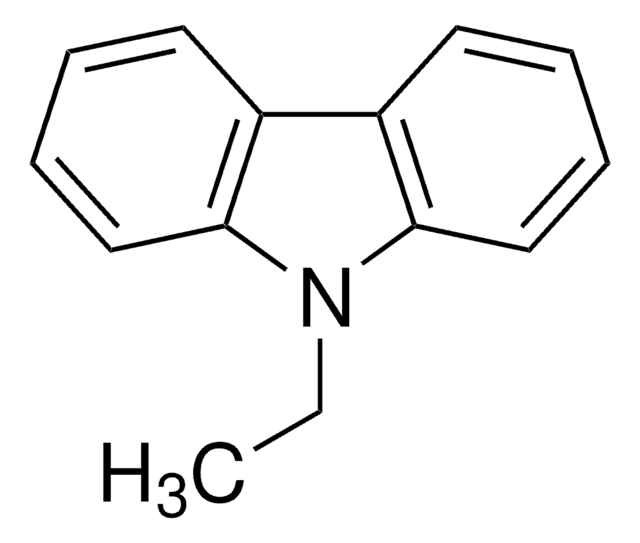
N-Methyldiphenylamine is an aromatic tertiary amine. It undergoes transformation to N-methylcarbazole (C) via a photochemical reaction.
Application
N-Methyldiphenylamine may be used for the synthesis of phosphonium ion salts. It may also be used as a starting reagent for the preparation of bis(4-carboxyphenyl)-N-methylamine (H2CPMA).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tae Kyung Kim et al.
Inorganic chemistry, 52(2), 589-595 (2012-12-29)
A luminescent lithium metal-organic framework (MOF) is constructed from the solvothermal reaction of Li(+) and a well-designed organic ligand, bis(4-carboxyphenyl)-N-methylamine (H(2)CPMA). A Li-based MOF can detect an explosive aromatic compound containing nitro groups as an explosophore, by showing a dramatic
Reaction patterns and kinetics of the photoconversion of N-methyldiphenylamine to N-methylcarbazole.
Forster EW, et al.
Journal of the American Chemical Society, 95(10), 3108-3115 (1973)
Frustrated Lewis pair-mediated C?O or C?H bond activation of ethers.
Holthausen MH, et al.
Chemical Communications (Cambridge, England), 50(70), 10038-10040 (2014)
Natália M Monezi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 173, 462-467 (2016-10-08)
The distinct thermochromism observed in solutions containing N,N-dimethylaniline (DMA) and N,N-diethylaniline (DEA) and SO2 was investigated by resonance Raman spectroscopy in a wide range of temperatures. The results indicate in addition to the charge transfer (CT) complexes DMA-SO2 and DEA-SO2
Minoru Yamaji et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 14(9), 1673-1684 (2015-07-07)
Photochemical processes of 4-tert-butyl-4'-methoxydibenzoylmethane (Avobenzone, AB), 4-phenylbenzoylbenzoyl-, 4-phenylbenzoyl-2'-furanyl- and 4-phenylbenzoyl-2'-thenoylmethanes (PB@Ph, PB@F and PB@T, respectively) substituted with Br and Cl at the C2 position were studied by stationary and laser flash photolyses in solution. The absorption spectral features showed that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service