488216
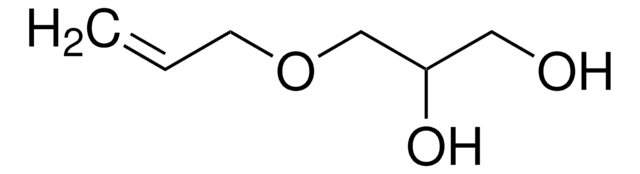
3,4-Dihydroxy-1-butene
≥99%
Synonym(s):
3-Butene-1,2-diol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH2=CHCH(OH)CH2OH
CAS Number:
Molecular Weight:
88.11
Beilstein:
1633578
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
bp
195 °C/733 mmHg (lit.)
density
1.047 g/mL at 25 °C (lit.)
SMILES string
OCC(O)C=C
InChI
1S/C4H8O2/c1-2-4(6)3-5/h2,4-6H,1,3H2
InChI key
ITMIAZBRRZANGB-UHFFFAOYSA-N
General description
3,4-Dihydroxy-1-butene, also known as 3-butene-1,2-diol (BDdiol), is a metabolite of 1,3-butadiene. It forms the precursor for synthesizing different chiral building blocks. BDdiol can undergo oxidation to form hydroxymethylvinyl ketone (HMVK). 1,2-epoxy-3-butene (EB) on hydrolysis in the presence of epoxide hydrolases (EH) forms BDdiol.
Application
3,4-Dihydroxy-1-butene can be used:
- As a reactant to synthesize cyclic organic carbonates by continuous flow procedure.
- To prepare substituted oxazolidinone ligands used to target medicinally relevant RNAs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3-Butene-1, 2-diol: An attractive precursor for the synthesis of enantiomerically pure organic compounds.
Rao AVR, et al.
Tetrahedron, 45(22), 7031-7040 (1989)
Metabolism of 1,3-butadiene to toxicologically relevant metabolites in single-exposed mice and rats.
Johannes Georg Filser et al.
Chemico-biological interactions, 166(1-3), 93-103 (2006-04-18)
1,3-Butadiene (BD) was carcinogenic in rodents. This effect is related to reactive metabolites such as 1,2-epoxy-3-butene (EB) and especially 1,2:3,4-diepoxybutane (DEB). A third mutagenic epoxide, 3,4-epoxy-1,2-butanediol (EBD), can be formed from DEB and from 3-butene-1,2-diol (B-diol), the hydrolysis product of
R J Krause et al.
Drug metabolism and disposition: the biological fate of chemicals, 25(8), 1013-1015 (1997-08-01)
Incubations of butadiene monoxide (BMO) with mouse, rat, and human liver microsomes or cDNA-expressed human microsomal epoxide hydrolase led to 3-buten-1,2-diol (BDD) detection; the BDD peak exhibited a GC/MS fragmentation pattern similar to that of reference material. Incubations with rat
M W Powley et al.
Carcinogenesis, 26(9), 1573-1580 (2005-05-13)
1,3-Butadiene (BD) is a confirmed rodent carcinogen and a suspect human carcinogen that forms mutagenic epoxide metabolites during biotransformation. Species differences in the roles of individual DNA reactive intermediates in BD mutagenicity and carcinogenicity are not completely understood. Evidence suggests
Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions
Gerardy R, et al.
Catalysis Science & Technology, 9(24), 6841-6851 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service