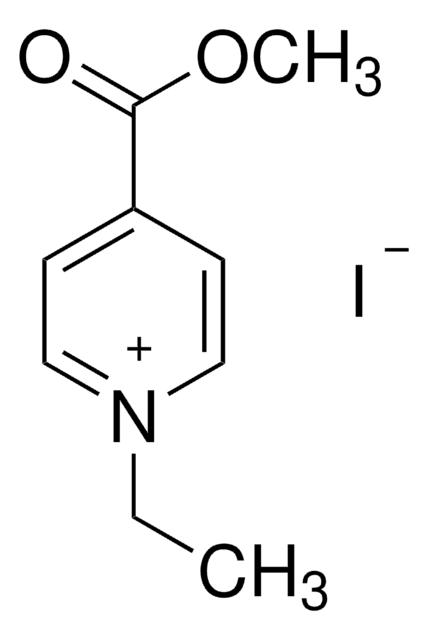
47714
4-Formyl-1-methylpyridinium benzenesulfonate
≥95.0%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H13NO4S
CAS Number:
Molecular Weight:
279.31
Beilstein:
5695963
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
impurities
≤2.0% water
mp
~95 °C
functional group
aldehyde
sulfonic acid
SMILES string
[H]C(=O)c1cc[n+](C)cc1.[O-]S(=O)(=O)c2ccccc2
InChI
1S/C7H8NO.C6H6O3S/c1-8-4-2-7(6-9)3-5-8;7-10(8,9)6-4-2-1-3-5-6/h2-6H,1H3;1-5H,(H,7,8,9)/q+1;/p-1
InChI key
HSVLGIFAXFDLMU-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
4-Formyl-1-methylpyridinium benzenesulfonate is a pyridinium salt widely used for the conversion of primary amines to the carbonyl compounds like aldehydes and ketones. The reaction conditions are mild, suitable for compounds with sensitive functional groups thereby providing an efficient alternative for such transformations.
Application
4-Formyl-1-methylpyridinium benzenesulfonate may be used as a reagent in the synthesis of the following:
- tetrazolic analogs of chalcones
- (+)-ferruginol
- Ecteinascidin 743
- Galipea alkaloids
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jinchun Chen et al.
Journal of the American Chemical Society, 128(1), 87-89 (2006-01-05)
A convergent total synthesis of ecteinascidin 743 is realized from five building blocks of almost equal size. It takes 23 steps from l-3-hydroxy-4-methoxy-5-methyl phenylalanol (5) with an overall yield of 3%.
Zacharias Amara et al.
Natural product reports, 30(9), 1211-1225 (2013-07-31)
This review focuses on recent applications of the aza-Michael reaction in alkaloids total synthesis with a special emphasis on stereoselectivity. The report highlights achievements and challenges over the past five years and describes stereoselective intra- and inter-molecular conjugate addition of
Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine.
Gonzalez MA and Perez-Guaita D.
Tetrahedron, 68(47), 9612-9615 (2012)
Ornella Mesenzani et al.
Bioorganic & medicinal chemistry letters, 21(2), 764-768 (2010-12-21)
In the chalcone scaffold, it is thought that the double bond is an important structural linker but it is likely not essential for the interaction with tubulin. Yet, it may be a potential site of metabolic degradation and interaction with
Mild and simple biomimetic conversion of amines to carbonyl compounds.
Buckley TF and Rapoport H
Journal of the American Chemical Society, 104(16), 4446-4450 (1982)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service