All Photos(1)
About This Item
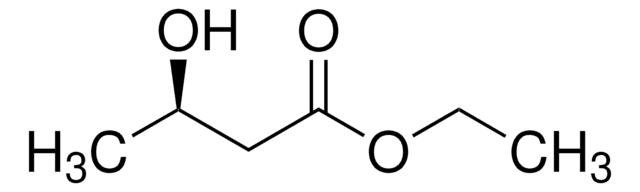
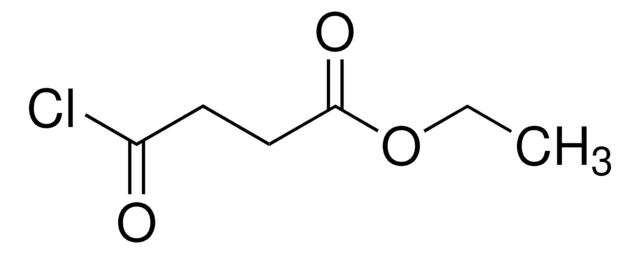
Linear Formula:
ClCH2CH(OH)CH2CO2C2H5
CAS Number:
Molecular Weight:
166.60
Beilstein:
4657170
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
optical activity
[α]23/D −14°, neat
optical purity
ee: 97% (GLC)
refractive index
n20/D 1.453 (lit.)
bp
93-95 °C/5 mmHg (lit.)
density
1.19 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C[C@H](O)CCl
InChI
1S/C6H11ClO3/c1-2-10-6(9)3-5(8)4-7/h5,8H,2-4H2,1H3/t5-/m0/s1
InChI key
ZAJNMXDBJKCCAT-YFKPBYRVSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl (S)-4-chloro-3-hydroxybutanoate is a chiral intermediate generally used to prepare atorvastatin, a cholesterol-lowering drug.
Application
Ethyl (S)-(−)-4-chloro-3-hydroxybutyrate can be used as a starting material in the synthesis of 4-amino-3-hydroxybutyric acid, a compound of pharmacological importance.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Efficient Asymmetric Synthesis of Ethyl (S)-4-Chloro-3-hydroxybutyrate Using Alcohol Dehydrogenase Sm ADH31 with High Tolerance of Substrate and Product in a Monophasic Aqueous System
Yang Z, et al.
Organic Process Research & Development, 24(6), 1068?1076-1068?1076 (2020)
Cloning, expression and characterization of a highly active alcohol dehydrogenase for production of ethyl (S)-4-chloro-3-hydroxybutyrate
Zhu Y-H, et al.
Indian Journal of Microbiology, 59(2), 225-233 (2019)
Short synthesis of (R)-and (S)-4-amino-3-hydroxybutyric acid (GABOB).
Tiecco M, et al.
Synthesis, 2005(04), 579-582 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service