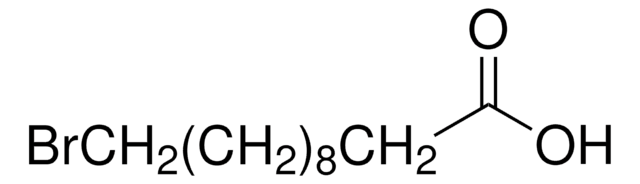447463
Methyl 11-bromoundecanoate
95%
Synonym(s):
11-Bromoundecanoic acid methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Br(CH2)10CO2CH3
CAS Number:
Molecular Weight:
279.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.465 (lit.)
bp
115 °C/0.04 mmHg (lit.)
density
1.157 g/mL at 25 °C (lit.)
functional group
bromo
ester
SMILES string
COC(=O)CCCCCCCCCCBr
InChI
1S/C12H23BrO2/c1-15-12(14)10-8-6-4-2-3-5-7-9-11-13/h2-11H2,1H3
InChI key
HFNPVFKUZYCDIB-UHFFFAOYSA-N
Related Categories
Application
Methyl 11-bromoundecanoate can be used as a reactant to synthesize:
- Methyl 11-(2,5-dibromophenoxy)undecanoate, which is employed as a precursor to prepare acetylenic cyclophanes.
- Methyl 11-[(1-phenyl-1H-tetrazol-5-yl)thio]undecanoate, a key intermediate applicable in the synthesis of emmyguyacins side chain.
- Betain derivatives of 11-bromoundecanoic acid, as potential microbial agents.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, characterization, antimicrobial and anti-biofilm activity of a new class of 11-bromoundecanoic acid-based betaines
Yasa SR, et al.
Medicinal Chemistry Research, 26(10), 2592-2601 (2017)
A Three Step Synthesis of 11-Cycloheptylundecanoic Acid, a Component of the Thermoacidophile Alicyclobacillus cycloheptanicus.
Hassarajani SA and Mamdapur VR.
Molecules (Basel), 3(2), 41-43 (1998)
Collins et al.
Organic letters, 2(20), 3189-3192 (2000-09-29)
The synthesis of a series of novel acetylenic cyclophanes is described. X-ray crystallographic analysis of the core structure revealed a twisted conformation with helical chirality. Preliminary results suggest that these cyclophanes, with appropriate functionality, have the potential to act as
Studies on ω-Oxidation of Fatty Acids in vitro.
KAMEI S, et al.
Journal of Biochemistry, 56(1), 72-76 (1964)
Santanu Jana et al.
Organic letters, 20(21), 6938-6942 (2018-10-24)
Fungal glycolipids emmyguyacins A and B inhibit the pH-dependent conformational change of hemaglutinin A during replication of the Influenza virus. Herein, we report the first total synthesis and structure confirmation of emmyguyacins A and B. Our efficient route, which involves
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service