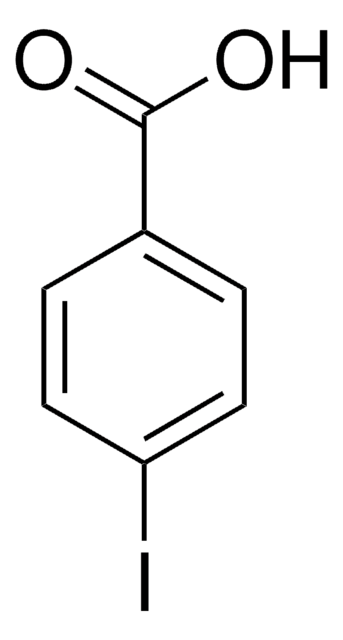
40288
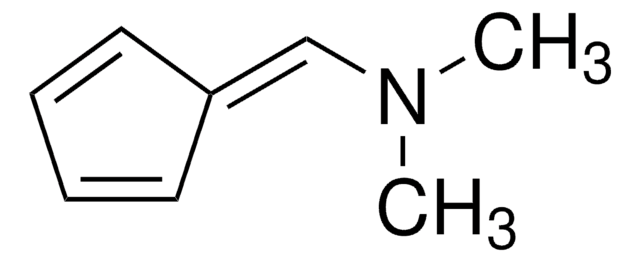
6,6-Dimethylfulvene
≥95%
Synonym(s):
5-(1-Methylethylidene)-1,3-cyclopentadiene, 5-Isopropylidene-1,3-cyclopentadiene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H10
CAS Number:
Molecular Weight:
106.17
Beilstein:
1616308
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
liquid
refractive index
n20/D 1.548 (lit.)
n20/D 1.548
bp
76-77 °C/50 mmHg (lit.)
density
0.881 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
C\C(C)=C1/C=CC=C1
InChI
1S/C8H10/c1-7(2)8-5-3-4-6-8/h3-6H,1-2H3
InChI key
WXACXMWYHXOSIX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6,6-Dimethylfulvene [5-(1-methylethylidene)-1,3-cyclopentadiene] is a nonaromatic carbocyclic analog of isopropylbenzene. 6,6-Dimethylfulvene reacts with 2,2-bis(trifluoromethyl)-1,1-dicyanoethylene (BTF; 1) to afford the expected Diels-Alder cycloadduct, 7-(1-methylethylidene)-3,3-bis(trifluoromethyl)bicyclo[2.2.1]hept-5-ene-2,2-dicarbonitrile. Metal-free hydrogenation of 6,6-dimethylfulvene via frustrated Lewis pair (FLP) mediated triple domino reaction has been reported.The biotransformation of 6,6-dimethylfulvene by Pseudomonas putida RE213 has been studied.Cycloaddition of 6,6-dimethylfulvene with benzynes has been reported.
6,6-Dimethylfulvene undergoes nucleophilic reaction with lithium dichloromethide in the presence of THF at -75°C, selectively at its exocyclic double bond.
Application
6,6-Dimethylfulvene may be employed in the following studies:
- One-pot synthesis of ansa-metallocenes.
- Synthesis of endo and exo-adducts with maleic anhydride.
- Synthesis of fulvenols or the corresponding trimethylsilyl ethers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
109.4 °F - closed cup
Flash Point(C)
43 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cycloaddition of 6, 6-Dimethylfulvene with Benzynes1.
Muneyuki R and Tanida H.
The Journal of Organic Chemistry, 31(6), 1988-1990 (1966)
One-Step Synthesis of Organolanthanide (II) Complexes from the Metal.
Recknagel A and Edelmann FT.
Angewandte Chemie (International Edition in English), 30(6), 693-694 (1991)
Carbenic philicity: 6, 6-dimethylfulvene as an indicator substrate.
Moss RA, et al.
Journal of the American College of Cardiology, 103(9), 2413-2415 (1981)
The Diels-Alder Reaction and the Rearrangement of the Maleic Anhydride Adducts of 6, 6-Dimethylfulvene.
Craig D, et al.
Journal of the American Chemical Society, 76(18), 4573-4575 (1954)
Sergej Tamke et al.
Organic & biomolecular chemistry, 12(45), 9139-9144 (2014-10-09)
The frustrated Lewis pair (FLP) mediated hydrosilylation of pentafulvenes is described yielding allyl silanes with high regioselectivity in excellent yields. While phenyl substituted allyl silanes undergo B(C6F5)3-mediated rearrangement to vinyl silanes, dimethyl derivatives experience FLP-catalyzed hydrogenation followed by an unprecedented
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service