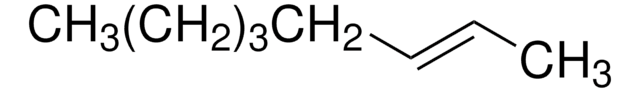All Photos(1)
About This Item
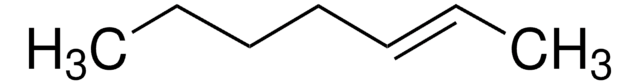
Linear Formula:
CH3CH2CH2CH=CHCH2CH2CH3
CAS Number:
Molecular Weight:
112.21
Beilstein:
1719104
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
≥90%
form
liquid
refractive index
n20/D 1.412 (lit.)
bp
122-123 °C (lit.)
solubility
water: insoluble
density
0.715 g/mL at 25 °C (lit.)
SMILES string
[H]\C(CCC)=C(\[H])CCC
InChI
1S/C8H16/c1-3-5-7-8-6-4-2/h7-8H,3-6H2,1-2H3/b8-7+
InChI key
IRUCBBFNLDIMIK-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
trans-4-Octene is an acyclic olefin. Its synthesis has been reported. Its Raman spectra has been reported. Rate constant of the gas-phase reactions of trans-4-octene with hydroxyl radicals and ozone has been investigated. Its enantiomeric epoxidation has been studied. Its isomerization into isomeric octenes has been studied.
Application
trans-4-Octene may be used in the synthesis of the following:
- n-nonanal
- aliphatic unsaturated polyesters
- 4-isopropyloctane
- diasterioisomeric 4-(difluoroiodomethyl)-5-iodooctane
- siliranes
- m-dioxanes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A convenient, mild method for the cyclization of 3-and 4-arylalkanoic acids via their trifluoromethanesulfonic anhydride derivatives
Hulin B and Koreeda M.
The Journal of Organic Chemistry, 49(1), 207-209 (1984)
Synthesis and characterization of aliphatic unsaturated polyesters from trans-4-octene-1, 8-dioic and trans-3-hexene-1, 6-dioic acids.
Maglio G et al.
Eur. Polymer J., 15(7), 695-699 (1979)
Synthesis, physicochemical studies and aerobic enantioselective epoxidation of non functionalized olefins catalyzed by new Co (II) chiral salen complexes.
Kureshy RI et al.
J. Mol. Catal. A: Chem., 121(1), 25-31 (1997)
The Stereochemistry of the Debromination of Vicinal Dibromides by Metals1.
Schubert WM, et al.
Journal of the American Chemical Society, 74(18), 4590-4592 (1952)
Raman Spectra of Hydrocarbons I. 1-Octene, cis+ trans 2-Octene, trans-3-Octene, trans-4-Octene, 4-Octyne, and 1-Octyne.
Cleveland FF.
J. Chem. Phys. , 11(1), 1-6 (1943)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service