378755
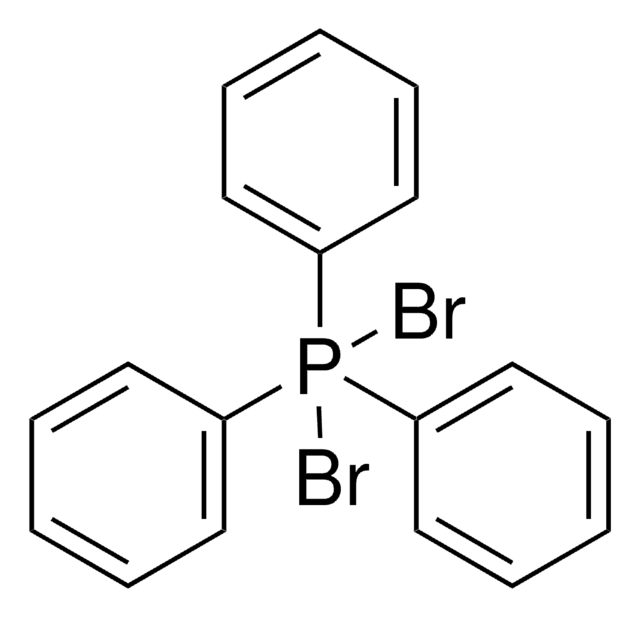
Dichlorotriphenylphosphorane
95%
Synonym(s):
Triphenylphosphine dichloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(C6H5)3PCl2
CAS Number:
Molecular Weight:
333.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
85 °C (dec.) (lit.)
functional group
phosphine
SMILES string
ClP(Cl)(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C18H15Cl2P/c19-21(20,16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
InChI key
ASWXNYNXAOQCCD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dichlorotriphenylphosphorane (Triphenylphosphine dichloride, PPh3Cl2) is a phosphorous halide. Stability and reactivity of dichlorotriphenylphosphorane have been studied by solid state 31P NMR spectroscopy. It participates in the conversion of carboxylic esters to acid halides and mechanism of this cleavage has been investigated. Modified method has been proposed in which dichlorotriphenylphosphorane participates in the synthesis of tertiary amides.
Application
Dichlorotriphenylphosphorane may be used in the synthesis of the following:
- tertiary benzanilide derivatives
- N-phosphodipeptides
- dialkyl phosphorochloridites based on the reaction of trialkyl phosphites
- naphthalene ring-based calix[3]amide, via reaction with 6-amino-2-naphthoic acid
- C2v-symmetrical cyclic tetramers of aromatic amides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Eye Dam. 1 - Flam. Sol. 2 - Skin Corr. 1B
Storage Class Code
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cleavage of carboxylic acid esters to acid chlorides with dichlorotriphenylphosphorane.
Burton DKJ and Koppes WM.
The Journal of Organic Chemistry, 40(21), 3026-3032 (1975)
Nina C Gonnella et al.
Magnetic resonance in chemistry : MRC, 47(6), 461-464 (2009-03-07)
Solid state (31)P NMR spectroscopy was used to examine, monitor and quantify the compound integrity of the chemical reagent dichlorotriphenylphosphorane. Comparison was also made with solution (31)P NMR spectra which showed that this highly reactive species could be observed in
Construction of anomalously bent biphenyl structure using conformational properties of calix [4] amide.
Tominaga M, et al.
Tetrahedron Letters, 47(52), 346-350 (2006)
Synthesis of N-Phosphopeptides Coupled by Dichlorotriphenylphosphorane.
Dong S-Z, et al.
Synthetic Communications, 31(13), 2067-2075 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service