All Photos(1)
About This Item
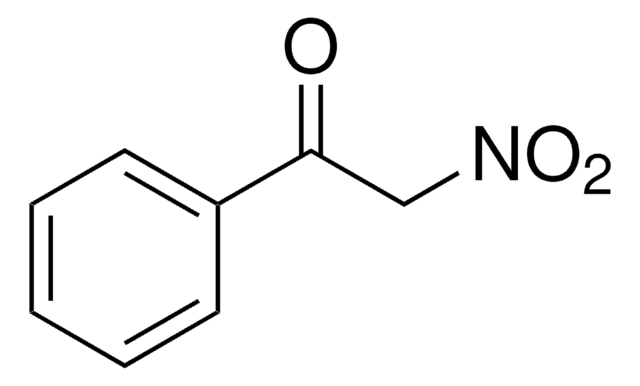
Linear Formula:
NO2CH2COOCH3
CAS Number:
Molecular Weight:
119.08
Beilstein:
1758670
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.425 (lit.)
bp
195-198 °C (lit.)
density
1.294 g/mL at 25 °C (lit.)
functional group
amine
ester
nitro
SMILES string
COC(=O)C[N+]([O-])=O
InChI
1S/C3H5NO4/c1-8-3(5)2-4(6)7/h2H2,1H3
InChI key
ALBSWLMUHHZLLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Methyl nitroacetate may be used:
- in the preparation of aromatic and heteroaromatic linear (E)-α-nitro-arylpentenoates
- to generate phenyliodonium ylide, used in the highly enantioselective and diastereoselective Cu(I)-catalyzed cyclopropanation of alkenes
- in the preparation of methyl (Z)-2-nitro-3-(4-nitrophenyl)-2-propenoate, via reaction with 4-nitrobenzylideneaniline
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
221.0 °F - closed cup
Flash Point(C)
105 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Andrew J Young et al.
Journal of the American Chemical Society, 130(43), 14090-14091 (2008-10-04)
The first electrophilic Pd(II)-catalyzed allylic C H alkylation is reported, providing a novel method for formation of sp3-sp3 C C bonds directly from C H bonds. A wide range of aromatic and heteroaromatic linear (E)-alpha-nitro-arylpentenoates are obtained as single olefin
Experimental and theoretical studies of Diels-Alder reaction between methyl (Z)-2-nitro-3-(4-nitrophenyl)-2-propenoate and cyclopentadiene.
Jasinski R, et al.
Monatshefte fur Chemie / Chemical Monthly, 144(3), 327-335 (2013)
Benoît Moreau et al.
Journal of the American Chemical Society, 127(51), 18014-18015 (2005-12-22)
A highly enantioselective (up to 97.5% ee) and diastereoselective (95:5 dr trans/cis) Cu(I)-catalyzed cyclopropanation of alkenes using phenyliodonium ylide generated in situ from iodosobenzene and methyl nitroacetate is reported. The cyclopropanation took place with high enantioselectivity for a wide range
[Experimental establishment of the maximum permissible concentration of the methyl and ethyl esters of nitroacetic acid].
G I Sidorin et al.
Gigiena truda i professional'nye zabolevaniia, (7)(7), 49-51 (1980-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service