337773
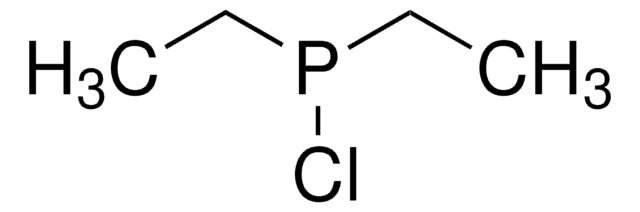
Chlorodiisopropylphosphine
96%
Synonym(s):
Diisopropylphosphine chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)2CH]2PCl
CAS Number:
Molecular Weight:
152.60
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
reaction suitability
reagent type: ligand
reaction type: Arylations
refractive index
n20/D 1.475 (lit.)
bp
69 °C/33 mmHg (lit.)
density
0.959 g/mL at 25 °C (lit.)
functional group
phosphine
SMILES string
CC(C)P(Cl)C(C)C
InChI
1S/C6H14ClP/c1-5(2)8(7)6(3)4/h5-6H,1-4H3
InChI key
JZPDBTOWHLZQFC-UHFFFAOYSA-N
Application
Chlorodiisopropylphosphine can be used:
- To synthesize p-styryldiisopropylphosphine by reacting with 4-chlorostyrene via Grignard reaction.
- As a phosphination reagent in combination with [Cp2Zr(1-butene)(DMAP)] (Cp=cyclopentadienyl; DMAP= 4-(dimethylamino)pyridine)) for the zirconophosphination of alkynes to form zirconoalkenylphosphines.
- A luminescent mixed-donor platinum POCN pincer complex via cyclometalation process.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F - closed cup
Flash Point(C)
4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, characterization and use of reactive polymers with diisopropylphenylphosphine groups.
Licea-Claverie A, et al.
Polymer Bull., 39(5), 551-557 (1997)
Metallophosphination of alkynes: efficient synthesis of ?-functionalized alkenylphosphines.
Xi C, et al.
Organometallics, 26(4), 1084-1088 (2007)
A luminescent Pt-POCN pincer complex via direct cyclometalation.
Lavelle K B, et al.
Journal of Organometallic Chemistry, 785, 100-105 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service