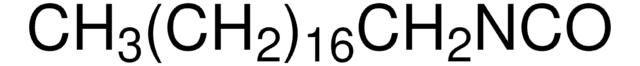All Photos(2)
About This Item
Linear Formula:
CH3(CH2)7NCO
CAS Number:
Molecular Weight:
155.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.432 (lit.)
bp
200-204 °C (lit.)
density
0.88 g/mL at 25 °C (lit.)
functional group
amine
isocyanate
SMILES string
CCCCCCCCN=C=O
InChI
1S/C9H17NO/c1-2-3-4-5-6-7-8-10-9-11/h2-8H2,1H3
InChI key
DYQFCTCUULUMTQ-UHFFFAOYSA-N
Related Categories
General description
Octyl isocyanate was reported to inactivate serine proteinase, chymotrypsin.
Application
Octyl isocyanate was used to suppress side reactions such as backbiting or chain transfer reaction during polymerization reactions. It was also used in the synthesis of:
- low molar mass organogelator containing 2-(2′-hydroxyphenyl)benzoxazole (HPB) unit with long alkyl chain
- N-octylurea
- N-cyclopropyl-N′-octylurea
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
159.8 °F - closed cup
Flash Point(C)
71 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W E Brown et al.
Science (New York, N.Y.), 174(4009), 608-610 (1971-11-05)
Alkyl isocyanates react specifically with the two serine proteinases, chymotrypsin and elastase, to yield inactive enzyme derivatives containing 1 male of reagent per mole of enzyme. Octyl isocyanate inactivates chymotrypsin only, while butyl isocyanate inactivates both enzymes but shows greater
Tae Hyeon Kim et al.
Journal of nanoscience and nanotechnology, 10(10), 6929-6933 (2010-12-09)
A low molar mass organogelator 1 containing 2-(2'-hydroxyphenyl)benzoxazole (HPB) unit with long alkyl chain was synthesized by the reaction with HPB and octyl isocyanate in THF at room temperature. A new chelate-based organogelator 1-Zn(II) was prepared with the reaction of
C Morisseau et al.
Chemical research in toxicology, 14(4), 409-415 (2001-04-17)
The microsomal epoxide hydrolase (mEH) plays a significant role in the metabolism of xenobiotics such as polyaromatic toxicants. Additionally, polymorphism studies have underlined a potential role of this enzyme in relation to several diseases, such as emphysema, spontaneous abortion, and
Anionic polymerization of isocyanates with optical functionalities.
Shin YD, et al.
Polymer, 42(19), 7979-7985 (2001)
Isocyanate binding to yeast glutathione reductase measured by fluorescence spectroscopy.
K J Baylor et al.
Biochemical Society transactions, 25(1), 47S-47S (1997-02-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service