All Photos(1)
About This Item
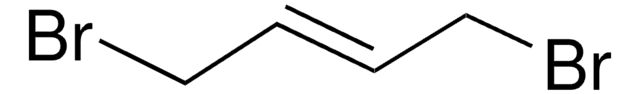
Linear Formula:
ClCH2CH=CHCH2Cl
CAS Number:
Molecular Weight:
125.00
Beilstein:
1719693
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
10 mmHg ( 20 °C)
Assay
98%
form
liquid
refractive index
n20/D 1.488 (lit.)
bp
74-76 °C/40 mmHg (lit.)
mp
1-3 °C (lit.)
density
1.183 g/mL at 25 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
ClC\C=C\CCl
InChI
1S/C4H6Cl2/c5-3-1-2-4-6/h1-2H,3-4H2/b2-1+
InChI key
FQDIANVAWVHZIR-OWOJBTEDSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans-1,4-Dichloro-2-butene may be used for the alkylation of adenine to 9-alkylpurines. It can react with diethyl malonate to form vinylcyclopropane derivative. It can also undergo asymmetric allylic alkylation with Grignard reagents in the presence of copper thiophene carboxylate catalyst.
Other Notes
85%, remainder predominantly cis isomer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
127.4 °F - closed cup
Flash Point(C)
53 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
High Diversity on Simple Substrates: 1, 4?Dihalo?2?butenes and Other Difunctionalized Allylic Halides for Copper?Catalyzed SN2? Reactions.
Falciola C A, et al.
Chemistry?A European Journal , 14(34), 10615-10627 (2008)
Improved selectivity in the preparation of some 1, 1-difunctionalized 3-cyclopentenes. High yield synthesis of 3-cyclopentenecarboxylic acid.
Depres JP and Greene AE.
The Journal of Organic Chemistry, 49(5), 928-931 (1984)
S Phadtare et al.
Journal of medicinal chemistry, 30(2), 437-440 (1987-02-01)
Alkylation of adenine (5a) or 2-amino-6-chloropurine (5b) with excess trans-1,4-dichloro-2-butene (4), effected by K2CO3 in dimethyl sulfoxide or tetra-n-butylammonium fluoride in tetrahydrofuran, led in 90-95% regioselectivity to 9-alkylpurines 6a and 6b. The title compounds 2a and 2b were obtained by
S Phadtare et al.
Nucleic acids symposium series, (18)(18), 25-28 (1987-01-01)
Reaction of adenine (1a) or cytosine (1b) with excess 1,4-dichloro-2-butyne catalyzed by K2CO3 in (CH3)2SO gave the 4-chloro-2-butynyl derivatives 2a and 2b. The latter were converted to the 4-hydroxy-2-butynyl compounds 3a and 3b by refluxing in 0.1 M HCl. Isomerization
[Experimental data on a hygienic standard for 1,4-dichlorobutene-2 in the air of a work area].
M S Gizhlarian et al.
Gigiena truda i professional'nye zabolevaniia, (4)(4), 49-50 (1985-04-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service