All Photos(2)
About This Item
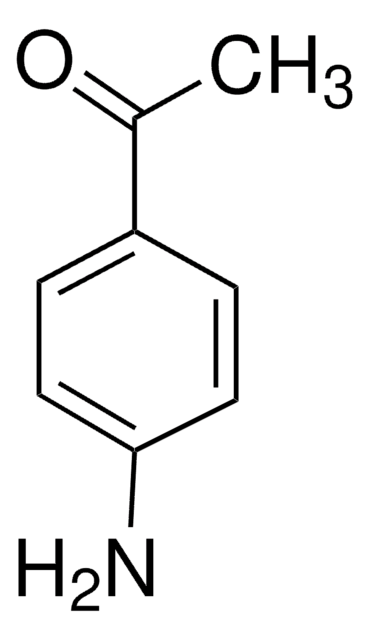
Linear Formula:
H2NC6H4CONH2
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
181-183 °C (lit.)
SMILES string
NC(=O)c1ccc(N)cc1
InChI
1S/C7H8N2O/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H2,9,10)
InChI key
QIKYZXDTTPVVAC-UHFFFAOYSA-N
Related Categories
Application
4-Aminobenzamide was used as a poly(ADP-ribose)polymerase (PADPRP) inhhibitor to study the death of target cells by cytotoxic effector cells using the nuclear enzyme poly(ADP-ribose)polymerase (PADPRP).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Burgman et al.
Radiation research, 119(2), 380-386 (1989-08-01)
The purpose of this study was to investigate possible involvement of poly(ADP-ribosyl)ation reactions in X-ray-induced cell killing, repair of potentially lethal damage (PLD), and formation and repair of radiation-induced DNA damage. As tools we used the inhibitors of poly(ADP-ribose)polymerase, 3-aminobenzamide
R J Baugh et al.
The Journal of biological chemistry, 275(37), 28826-28833 (2000-07-13)
The initiation of coagulation results from the activation of factor X by an enzyme complex (Xase) composed of the trypsin-like serine proteinase, factor VIIa, bound to tissue factor (TF) on phospholipid membranes. We have investigated the basis for the protein
S D Ray et al.
Molecular and cellular biochemistry, 218(1-2), 27-33 (2001-05-02)
Previous studies from our laboratories have linked the protective abilities of IH636 grape seed proanthocyanidin extract (GSPE) with inactivation of anti-apoptotic gene bcl-XL, and modification of several other critical molecular targets such as DNA-damage/DNA-repair, lipid peroxidation and intracellular Ca2+ homeostasis.
R Laffranchi et al.
Experimental cell research, 237(1), 217-222 (1998-01-07)
Interferon-gamma is among the cytokines which have been implicated as effector molecules of beta-cell destruction in autoimmune diabetes. Its mechanism of action is, however, largely unknown. In the present study rat pancreatic beta-cells, INS-1, were incubated with rat interferon-gamma (rIRN-gamma)
Jamie Lajiness et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 5(3), 216-226 (2009-05-16)
Imidazole and pyrrole-containing polyamides belong to an important class of compounds that can be designed to target specific DNA sequences, and they are potentially useful in applications of controlling gene expression. The extent of polyamide curvature is an important consideration
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service