264431
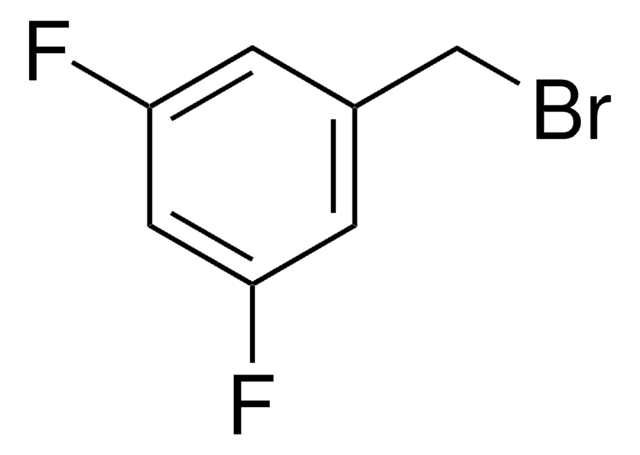
2,6-Difluorobenzyl bromide
97%
Synonym(s):
α-Bromo-2,6-difluorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H3CH2Br
CAS Number:
Molecular Weight:
207.02
Beilstein:
2083943
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
52-55 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1cccc(F)c1CBr
InChI
1S/C7H5BrF2/c8-4-5-6(9)2-1-3-7(5)10/h1-3H,4H2
InChI key
LSXJPJGBWSZHTM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,6-Difluorobenzyl bromide has been used:
- as reagent in alkylation of the quinazoline-2-thioxo-4-one
- in the synthesis of 1,3,5-triazine-2,4,6-triones
- in the preparation of new classes of inhibitors of bovine viral diarrhea virus (as a surrogate virus for hepatitis C virus)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Zhiqiang Guo et al.
Bioorganic & medicinal chemistry letters, 15(3), 693-698 (2005-01-25)
A convenient one-pot synthetic route was developed for the preparation of asymmetric 1,3-dialkyl-1,3,5-triazine-2,4,6-triones from readily available alkyl- or aryl-isocyanates, primary amines and N-chlorocarbonyl isocyanate in excellent yields. Subsequent alkylation with N-protected amino alcohols afforded the desired 1,3,5-triazine-2,4,6-triones in good yields.
Efficient solid-phase synthesis of quinazoline-2-thioxo-4-ones with SynPhase? lanterns.
Makino S, et al.
Tetrahedron Letters, 41(43), 8333-8337 (2000)
Gwang-Noh Ahn et al.
Lab on a chip, 19(20), 3535-3542 (2019-09-27)
Microreactors are emerging as an efficient, sustainable synthetic tool compared to conventional batch reactors. Here, we present a new numbering-up metal microreactor by integrating a flow distributor and a copper catalytic module for high productivity of a commercial synthetic drug.
Gerhard Puerstinger et al.
Bioorganic & medicinal chemistry letters, 16(20), 5345-5349 (2006-08-12)
A novel class of inhibitors of pestiviruses (5-substituted 2-phenyl-5H-imidazo[4,5-c]pyridines) is described. Modification of the substituent in position 5 resulted in analogues with high activity (EC(50)<100nM) and selectivity (SI>1000) against the pestivirus BVDV (bovine viral diarrhea virus).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service