263761
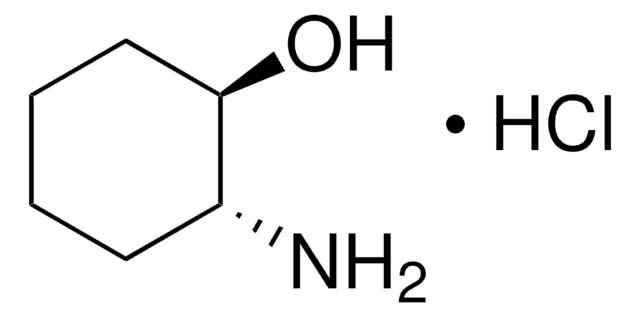
trans-4-Aminocyclohexanol hydrochloride
97%
Synonym(s):
trans-4-Hydroxycyclohexylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC6H10OH · HCl
CAS Number:
Molecular Weight:
151.63
Beilstein:
3909294
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
225-227 °C (lit.)
functional group
hydroxyl
SMILES string
Cl.N[C@H]1CC[C@H](O)CC1
InChI
1S/C6H13NO.ClH/c7-5-1-3-6(8)4-2-5;/h5-6,8H,1-4,7H2;1H/t5-,6-;
InChI key
RKTQEVMZBCBOSB-KYOXOEKESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
trans-4-Aminocyclohexanol hydrochloride has been used in the synthesis of:
- N-substituted 7-azabicyclo[2.2.1]heptanes via transannular nucleophilic displacement
- trans-4-methoxyoxalamido-1-cyclohexanol
- benzoxazine, required for the preparation of polybenzoxazine-silica hybrid nanocomposites
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Polybenzoxazine-silica (PBZ-SiO2) hybrid nanocomposites through in situ sol-gel method.
Devaraju S, et al.
Journal of Sol-Gel Science and Technology, 60(1), 33-40 (2011)
Synthesis and microbial hydroxylation of some azabicycloalkanes.
Olivo HF, et al.
Tetrahedron Letters, 39(11), 1309-1312 (1998)
D M Creasy et al.
Experimental and molecular pathology, 52(2), 155-169 (1990-04-01)
Male Wistar strain rats were fed a diet providing an intake of 0 or 400 mg cyclohexylamine (CHA)/kg body weight/day for 1, 3, 7, 9, or 13 weeks. At the end of the appropriate feeding period the rats were perfused-fixed
N N Polushin
Nucleic acids research, 28(16), 3125-3133 (2000-08-10)
Synthesis of new terminus modifiers, bearing, along with a phosphoramidite moiety, one, two or four methoxyoxalamido (MOX) precursor groups, is described. These modifiers are introduced onto the 5'-end of a synthetic oligodeoxyribonucleotide as the last step of an automated synthesis
Dwayne A Dias et al.
Organic letters, 11(16), 3694-3697 (2009-07-28)
The intramolecular variant of the homo-[3 + 2]-dipolar cycloaddition of nitrones (generated in situ from an aldehyde and a hydroxylamine) with donor-acceptor cyclopropanes allows for the efficient synthesis of bridged tetrahydro-1,2-oxazines. This domino sequence produces adducts amenable to reductive N-O
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service