257923
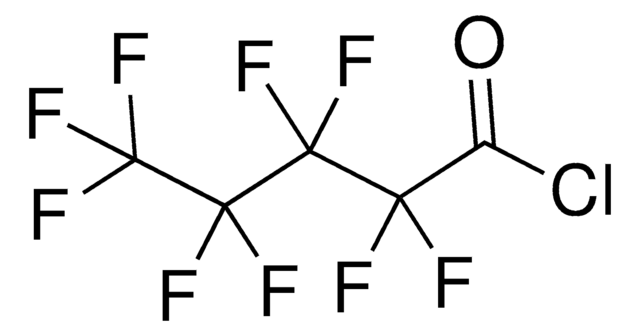
Perfluorobutyryl chloride
98%
Synonym(s):
Heptafluorobutyryl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3CF2CF2COCl
CAS Number:
Molecular Weight:
232.48
Beilstein:
1790269
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
7.22 psi ( 20 °C)
Assay
98%
form
liquid
refractive index
n20/D 1.3 (lit.)
bp
39 °C (lit.)
density
1.556 g/mL at 25 °C (lit.)
functional group
acyl chloride
storage temp.
2-8°C
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(Cl)=O
InChI
1S/C4ClF7O/c5-1(13)2(6,7)3(8,9)4(10,11)12
InChI key
WFELVFKXQJYPSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Perfluorobutyryl chloride has been used in the preparation of:
- ethylcellulose perfluorobutyrate, non-thrombogenic novel fluoropolymer for gas-exchange membranes
- 2-perfluoroalkylbenzothiazole
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R J Petersen et al.
Transactions - American Society for Artificial Internal Organs, 21, 242-248 (1975-01-01)
A novel fluoropolymer prepared from ethylcellulose by acylation with perfluorobutyryl chloride has been shown to be remarkably non-thrombogenic in vena cava and renal embolus ring implant tests. Gas permeabilities of this fluoropolymer are dufficient to make it highly attractive for
Synthesis of 2-and 2, 5-substituted benzoxazoles and benzothiazoles.
Soloski EJ, et al.
Journal of Fluorine Chemistry, 8(4), 295-304 (1976)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service