220884
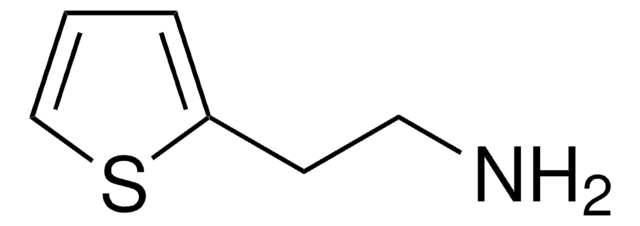
2-Thiophenemethylamine
96%
Synonym(s):
2-(Aminomethyl)thiophene
About This Item
Recommended Products
Quality Level
Assay
96%
form
liquid
liquid
refractive index
n20/D 1.5670 (lit.)
bp
95-99 °C/28 mmHg (lit.)
density
1.103 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
NCc1cccs1
InChI
1S/C5H7NS/c6-4-5-2-1-3-7-5/h1-3H,4,6H2
InChI key
FKKJJPMGAWGYPN-UHFFFAOYSA-N
General description
Application
- naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one
- fluorescent Pd2+ sensor, N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide
- Triazole-linked-thiopene conjugates for use as a biomimetic model for studies of metal detoxification and oxidative stress involving metallothionein
- Serotonin 5-HT1A receptor antagonists which have neuroprotective affects against ischemic cell damage
- Imidazole- and piperonyl-containing thiadiazoles and pyrimidines for use as inducible oxide synthase dimerization inhibitors
- Optoelectronic segmented polyurethanes
Reactant involved in:
- Studies of organocatalyzed asymmetric reductive amination of ketones
- Metal-free aerobic oxidative coupling of amines to imines
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service