All Photos(1)
About This Item
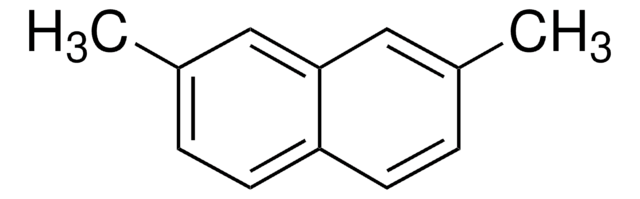
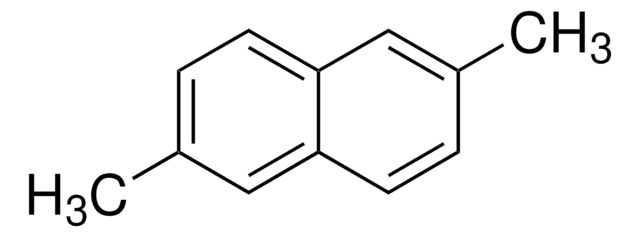
Linear Formula:
C10H6(OCH3)2
CAS Number:
Molecular Weight:
188.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
137-139 °C (lit.)
SMILES string
COc1ccc2ccc(OC)cc2c1
InChI
1S/C12H12O2/c1-13-11-5-3-9-4-6-12(14-2)8-10(9)7-11/h3-8H,1-2H3
InChI key
PPKHAIRFQKFMLE-UHFFFAOYSA-N
Application
2,7-Dimethoxynaphthalene was employed as matrix to investigate the structure of polymetallic porphyrins via matrix-assisted laser desorption/ionization. It was also used in the synthesis of:
- peri-aroylnaphthalene compounds via elective electrophilic aromatic aroylation
- 2-amino-1,2,3,4-tetrahydronaphthalene-6,7-diol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Iolinda Aiello et al.
Analytical chemistry, 76(20), 5985-5989 (2004-10-16)
2,7-Dimethoxynaphthalene (DMN) is proposed as matrix to investigate the structure of polymetallic porphyrins through matrix-assisted laser desorption/ionization tandem time-of-flight experiments. The peculiarity of DMN is represented by the formation of molecular radical cations and of some diagnostic fragments only. The
P Prince et al.
Acta crystallographica. Section C, Crystal structure communications, 45 ( Pt 8), 1255-1256 (1989-08-15)
C12H12O2, Mr = 188.2, orthorhombic, P2(1)2(1)2(1), a = 6.109 (3), b = 8.235 (2), c = 19.713 (3) A, V = 991.8 (9) A3, Z = 4, Dx = 1.260 g cm-3, lambda(Mo K alpha) = 0.71073 A, mu =
A Concise Synthesis of 2-Amino-1, 2, 3, 4-tetrahydronaphthalene-6, 7-diol ('6, 7-ADTN') from Naphthalene-2, 3-diol.
Goksu S, et al.
Helvetica Chimica Acta, 86(10), 3310-3313 (2003)
C L Perrin et al.
Journal of the American Chemical Society, 123(27), 6520-6526 (2001-07-06)
In solution, are the hydrogen bonds in monoprotonated N,N,N',N'-tetramethyl-1,8-naphthalenediamines single- or double-well? To answer this question, isotopic perturbation of equilibrium is applied to a mixture of -d(0), -d(3), -d(6), -d(9), and -d(12) isotopologs. The N-methyls of the 2,7-dimethoxy analogue show
Daichi Hijikata et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 11), o2902-o2903 (2010-01-01)
In the title mol-ecule {systematic name: [2,7-dimethoxy-8-(4-phenoxybenzoyl)naphthalen-1-yl](4-phenoxyphenyl)methan-one}, C(38)H(28)O(6), the 4-phen-oxy-benzoyl units adopt a syn orientation with respect to the naphthalene ring system. The inter-nal benzene rings, A and B, make dihedral angles of 86.72 (5) and 79.22 (5)° with the naphthalene ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service