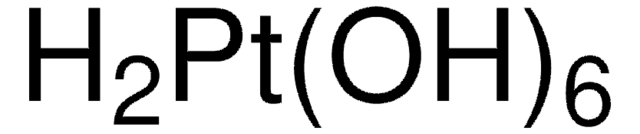206032
Platinum(IV) oxide
Synonym(s):
Adam’s catalyst, NSC 402624, Platinic oxide, Platinum dioxide
About This Item
Recommended Products
form
solid
composition
Pt, 81-83%
reaction suitability
core: platinum
reagent type: catalyst
greener alternative product characteristics
Designing Safer Chemicals
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
surface area
≥60 m2/g
mp
450 °C (lit.)
greener alternative category
, Aligned
SMILES string
O=[Pt]=O
InChI
1S/2O.Pt
InChI key
YKIOKAURTKXMSB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Hydrohydrazination reactions
- Oxidation of sugars
- Low-temperature oxidation of carbon monoxide
- Oxygen reduction reaction
- Hydrogenation reactions
- Hydrogenolysis of the cyclopropyl group into an isopropyl group
- Hydroxycyclopropanation reactions
For small scale and high throughput uses, product is also available as ChemBeads (928291)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Ox. Sol. 1
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis, and are some of the organic chemist’s most powerful tools for creating novel products. Below is a list of the most commonly used oxidizing and reducing agents currently available in our catalog.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service