156353
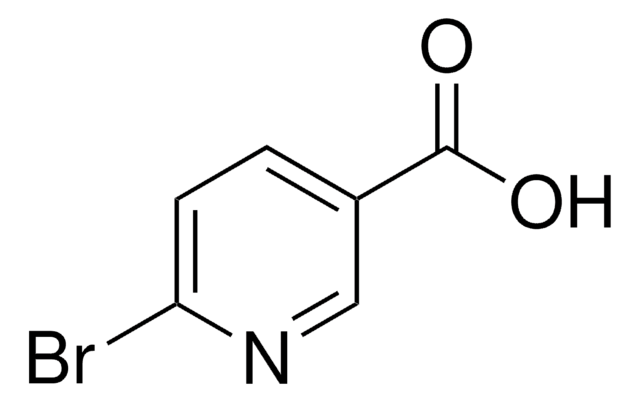
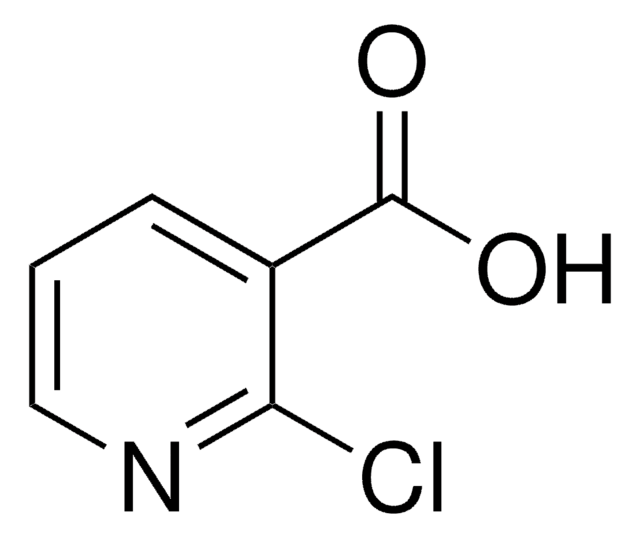
6-Chloropyridine-3-carboxylic acid
99%
Synonym(s):
6-Chloronicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H4ClNO2
CAS Number:
Molecular Weight:
157.55
Beilstein:
115993
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
190 °C (dec.) (lit.)
solubility
deionized water: soluble
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc(Cl)nc1
InChI
1S/C6H4ClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3H,(H,9,10)
InChI key
UAWMVMPAYRWUFX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Chloropyridine-3-carboxylic acid (6-chloronicotinic acid/6-CNA) has been used to study its photolytic and photocatalytic degradation. 6-CNA is a degradation product of neonicotinoid insecticides imidacloprid and acetamiprid and is known to appear in different environmental matrices. The product has been used as a media component during the isolation of 6-CNA degrading bacterial strain from imidacloprid-exposed soil samples.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mahrous M Kandil et al.
Journal of agricultural and food chemistry, 63(19), 4721-4727 (2015-05-02)
Thus far, only a small number and types of bacteria with limited ability in degrading imidacloprid have been reported. Also, genes regulating imidacloprid (IMDA) degradation have yet to be discovered. To study this in more detail, an enrichment technique was
Mehmet Karabacak et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(3), 876-883 (2008-03-25)
The experimental and theoretical study on the structures and vibrations of 6-chloronicotinic acid (6-CNA, C(6)H(4)ClNO(2)) are presented. The Fourier transform infrared spectra (4,000-50 cm(-1)) and the Fourier transform Raman spectra (3,500-50 cm(-1)) of the title molecule in solid phase have
A Segura Carretero et al.
Journal of chromatography. A, 1003(1-2), 189-195 (2003-08-06)
A method is described for the analysis of the insecticide imidacloprid [1-(6-chloro-3-pyridylmethyl)-N-nitroimidazolidin-2-ylideneamine] and its metabolite 6-chloronicotinic acid by micellar electrokinetic chromatography with diode-array detection at 270 and 227 nm, respectively. The best results were obtained using sodium dodecyl sulphate at
M D Gil García et al.
Journal of chromatography. A, 1147(1), 17-23 (2007-02-28)
The determination of imidacloprid and its main metabolite (6-chloronicotinic acid) in honeybees was performed by liquid chromatography with post-column photochemical derivatisation in alkaline medium and fluorescence detection. The compounds were extracted from honeybees with acetone under ultrasound conditions prior to
Romila Akoijam et al.
Environmental monitoring and assessment, 186(10), 5977-5984 (2014-06-04)
The metabolic degradation and persistence of imidacloprid in paddy field soil were investigated following two applications of imidacloprid at 20 and 80 g a.i. ha(-1) at an interval of 10 days. The soil samples were collected at various time intervals.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service