140856
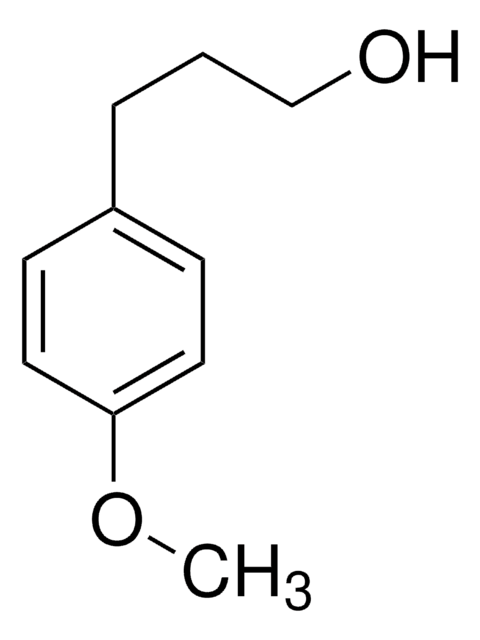
3-Phenyl-1-propanol
98%
Synonym(s):
3-Phenylpropyl alcohol, Hydrocinnamyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5(CH2)3OH
CAS Number:
Molecular Weight:
136.19
Beilstein:
1857542
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.526 (lit.)
bp
119-121 °C/12 mmHg (lit.)
mp
−18 °C (lit.)
density
1.001 g/mL at 20 °C (lit.)
functional group
hydroxyl
SMILES string
OCCCc1ccccc1
InChI
1S/C9H12O/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6,10H,4,7-8H2
InChI key
VAJVDSVGBWFCLW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Phenyl-1-propanol is a fragrance ingredient.
Application
3-Phenyl-1-propanol was used to study the hydrogenation of trans-cinnamaldehyde using water-soluble organometallic complexes. It was used as starting reagent during the enantioselective synthesis of (S)- and (R)-dapoxetine.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
248.0 °F - closed cup
Flash Point(C)
120 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The regioselective biphasic hydrogenation of trans-cinnaldehyde by meta sulfonatophenyl-diphenylphosphine (TPPMS) Ru (II) and Os (II) species. The influence of ionic strength, ligand tensoactivity and metal nature in the selective production of the unsaturated alcohol.
Lopez-Linares F, et al.
J. Mol. Catal. A: Chem., 145(1), 61-68 (1999)
S P Bhatia et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 49 Suppl 2, S246-S251 (2011-08-23)
A toxicologic and dermatologic review of 3-phenyl-1-propanol when used as a fragrance ingredient is presented. 3-Phenyl-1-propanol is a member of the fragrance structural group cinnamyl phenylpropyl compounds. The common characteristic structural element of cinnamyl phenylpropyl materials is an aryl substituted
Soyeong Kang et al.
The Journal of organic chemistry, 75(1), 237-240 (2009-12-05)
A highly efficient, enantioselective sequence has been developed for the synthesis of (S)- and (R)-dapoxetine. The pathways involve the intermediacy of the 6-membered-ring sulfamate esters 4, which were generated by Du Bois asymmetric C-H amination reactions of the prochiral sulfamate
Jörgen Samuelsson et al.
Journal of chromatography. A, 1217(46), 7215-7221 (2010-10-12)
The elution by characteristic points (ECP) method is a very rapid and precise method for determination of the phase system equilibrium of phase systems in broad solute concentration ranges. Thus, the method is especially suitable for rapid characterization of high
D Belsito et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 49 Suppl 2, S256-S267 (2011-08-09)
The cinnamyl phenylpropyl fragrance ingredients are a diverse group of chemical structures that have similar metabolic and toxicity profiles. A toxicological and dermatological review of these fragrance ingredients is presented. The common characteristic structural element of cinnamyl phenylpropyl materials is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service