All Photos(1)
About This Item
Empirical Formula (Hill Notation):
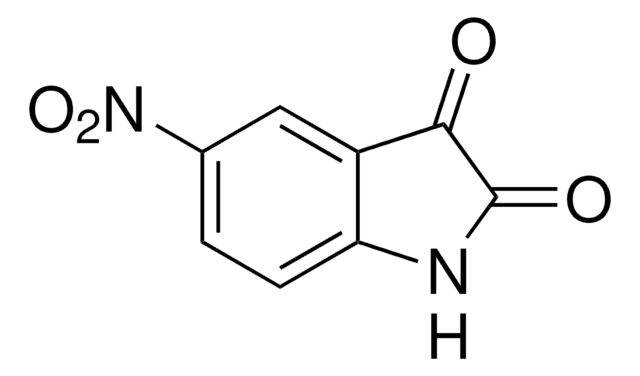
C8H4INO2
CAS Number:
Molecular Weight:
273.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
solid
mp
276-280 °C (lit.)
functional group
iodo
ketone
SMILES string
Ic1ccc2NC(=O)C(=O)c2c1
InChI
1S/C8H4INO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
InChI key
OEUGDMOJQQLVAZ-UHFFFAOYSA-N
Related Categories
General description
5-Iodoisatin undergoes condensation reaction with:
- phenol yields 5-iodophenolisatin
- malonic acid yields 6-iodo-2-quinolone-4-carboxylic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
CCCLXXXVII.-The relative stability of the quinolone and indolinone rings.
Aeschlimann JA.
Journal of the Chemical Society, 129, 2902-2912 (1926)
THE PREPARATION OF CERTAIN IODINATED DERIVATIVES OF PHENOLISATIN.
Sumpter WC.
Journal of the American Chemical Society, 54(9), 3766-3768 (1932)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)