13742
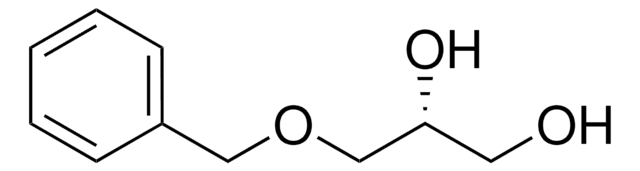
(±)-3-Benzyloxy-1,2-propanediol
≥97.0% (HPLC)
Synonym(s):
(±)-1-Benzylglycerol, (±)-Glycerol 1-benzyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2OCH2CHOHCH2OH
CAS Number:
Molecular Weight:
182.22
Beilstein:
3199937
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (HPLC)
refractive index
n20/D 1.533
density
1.140 g/mL at 20 °C (lit.)
functional group
ether
hydroxyl
phenyl
SMILES string
OCC(O)COCc1ccccc1
InChI
1S/C10H14O3/c11-6-10(12)8-13-7-9-4-2-1-3-5-9/h1-5,10-12H,6-8H2
InChI key
LWCIBYRXSHRIAP-UHFFFAOYSA-N
General description
(±)-3-Benzyloxy-1,2-propanediol undergoes enantioseparation by ligand exchange micellar electrokinetic chromatography using borate anion as a central ion.
Application
(±)-3-Benzyloxy-1,2-propanediol was used in capillary electrophoretic enantioseparation of vicinol diols using different β-cyclodextrin derivatives and borate. It was used in the synthesis and immobilization of 2,3-di-O-phytanyl-sn-glycerol-1-tetraethylene glycol-(3-trichloropropyl-silane) ether lipid (DPTTC) and 2,3-di-O-phytanyl-sn-glycerol-1-tetraethylene glycol-(3-chloro-dimethylpropyl-silane) ether lipid(DPTDC).
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vladimir Atanasov et al.
Biophysical journal, 89(3), 1780-1788 (2005-08-30)
Tethered membranes have been proven during recent years to be a powerful and flexible biomimetic platform. We reported in a previous article on the design of a new architecture based on the self-assembly of a thiolipid on ultrasmooth gold substrates
Shuji Kodama et al.
Electrophoresis, 26(20), 3884-3889 (2005-09-17)
Three compounds having 1,2-diol structure (1-phenyl-1,2-ethanediol, 3-phenoxy-1,2-propanediol, and 3-benzyloxy-1,2-propanediol) were enantioseparated by ligand exchange MEKC using (5S)-pinanediol (SPD) as a chiral selector and borate anion as a central ion together with SDS. When (S)-1,2-propanediol, (S)-1,2,4-butanetriol, or (S)-3-tert-butylamino-1,2-propanediol were used as
Capillary electrophoretic chiral resolution of vicinal diols by complexation with borate and cyclodextrin: Comparative studies on different cyclodextrin derivatives.
Schmid MG, et al.
Chirality, 9(2), 153-156 (1997)
Sean T Zuckerman et al.
Biomaterials, 30(23-24), 3825-3833 (2009-05-16)
Mass spectrometry is a powerful proteomic tool enabling researchers to survey the global proteome of a cell. This technique has only recently been employed to investigate cell-material interactions. We had previously identified material scarcity and limited adherent cells as challenges
Vincent J Huber et al.
Bioorganic & medicinal chemistry, 17(1), 411-417 (2008-01-10)
The in vitro inhibitory effects and in silico docking energies of 18 compounds with respect to aquaporin 4 (AQP4) were investigated. More than half of the compounds tested showed inhibitory activity in the in vitro functional assay and included the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service