136727
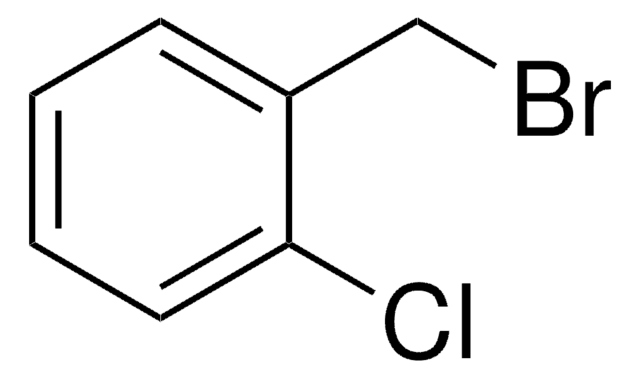
3-Chlorobenzyl bromide
97%
Synonym(s):
α-Bromo-3-chlorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4CH2Br
CAS Number:
Molecular Weight:
205.48
Beilstein:
636504
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.588 (lit.)
bp
109-110 °C/12 mmHg (lit.)
density
1.565 g/mL at 25 °C (lit.)
functional group
bromo
chloro
SMILES string
Clc1cccc(CBr)c1
InChI
1S/C7H6BrCl/c8-5-6-2-1-3-7(9)4-6/h1-4H,5H2
InChI key
LZIYAIRGDHSVED-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Chlorobenzyl bromide reacts with aminoethanol and NaH to yield aminoethyl 3-chlorobenzyl ether.
Application
3-Chlorobenzyl bromide was used in the synthesis of symmetrical and unsymmetrical benzyl thioethers. It was used as starting reagent during the synthesis of 1-(3-chlorobenzyl)-2-(pyrrolidin-1-ylmethyl)-1H-benzimidazole dihydrochloride.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective inactivation of monoamine oxidase B by aminoethyl 3-chlorobenzyl ether.
Ding CZ and Silverman RB.
Bioorganic & Medicinal Chemistry, 3(10), 2077-2078 (1993)
Convenient and robust one-pot synthesis of symmetrical and unsymmetrical benzyl thioethers from benzyl halides using thiourea.
Eccles KS, et al.
ARKIVOC (Gainesville, FL, United States), ix, 216-218 (2010)
Jason Z Vlahakis et al.
Bioorganic & medicinal chemistry, 21(21), 6788-6795 (2013-09-12)
Several analogs based on the lead structure of 1-(4-chlorobenzyl)-2-(pyrrolidin-1-ylmethyl)-1H-benzimidazole (clemizole) were synthesized and evaluated as novel inhibitors of heme oxygenase (HO). Many of the compounds were found to be potent and highly selective for the HO-2 isozyme (constitutive), and had
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service