126993
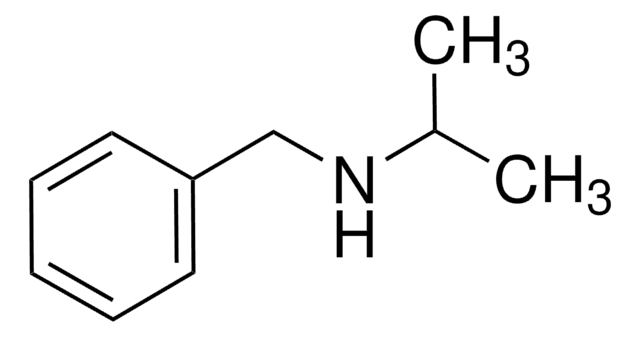
N-Ethylbenzylamine
97%
Synonym(s):
N-Benzylethylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2NHCH2CH3
CAS Number:
Molecular Weight:
135.21
Beilstein:
386023
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
191-194 °C (lit.)
density
0.909 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
CCNCc1ccccc1
InChI
1S/C9H13N/c1-2-10-8-9-6-4-3-5-7-9/h3-7,10H,2,8H2,1H3
InChI key
HVAAHUDGWQAAOJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Ethylbenzylamine has been used to demonstrate the reactivity of NDTE (2,5-dihydroxyphenylacetic acid, 2,5-bis-tetrahydropyranyl ether p-nitrophenyl ester) and HLTE (homogentisic gamma-lactone tetrahydropyranyl ether). It has also been used to prepare 2-[(N-Benzyl, N-ethyl)amino]derivative using 2-chloro-4H-pyrido[1,2-a]pyrimidin-4-one.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
156.2 °F - closed cup
Flash Point(C)
69 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Di Braccio et al.
Il Farmaco; edizione scientifica, 43(9), 705-723 (1988-09-01)
The N,N-disubstituted 4-amino-2H-pyrido[1,2-a]pyrimidin-2-ones (III) and isomer 2-amino-4H-pyrido[1,2-a]pyrimidin-4-ones (IV) were obtained from the reaction of 2-aminopyridine with the N,N-disubstituted ethyl malonamate/phosphorus oxychloride reagent (II), in refluxing 1,2-dichloroethane. 2-[(N-Benzyl, N-ethyl)amino]derivative (IV b) was also prepared in excellent yield by treating 2-chloro-4H-pyrido[1,2-a]pyrimidin-4-one (V)
M J Rose et al.
Analytical chemistry, 71(11), 2221-2230 (1999-06-15)
Two new reagents, NDTE (2,5-dihydroxyphenylacetic acid, 2,5-bis-tetrahydropyranyl ether p-nitrophenyl ester) and HLTE (homogentisic gamma-lactone tetrahydropyranyl ether), are described for the chemical derivatization of primary and/or secondary amines to form an electrochemically active product. These reagents undergo reaction with the aforementioned
Md Salman Shakil et al.
Biomedicines, 9(2) (2021-01-31)
Hydroxypyr(id)ones are a pharmaceutically important class of compounds that have shown potential in diverse areas of drug discovery. We investigated the 3-hydroxy-4-pyridones 1a-1c and 3-hydroxy-4-thiopyridones 1d-1f as well as their Ru(η6-p-cymene)Cl complexes 2a-2f, and report here the molecular structures of
Oh Kyung Choi et al.
Journal of hazardous materials, 403, 123636-123636 (2020-08-28)
Solvent extraction desalination (SED) is one of the liquid-liquid separation techniques that selectively uptake freshwater from high saline water, and then separate the absorbed freshwater from the solvent through temperature swing. This study evaluated the desalination performance of seven different
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service