123323
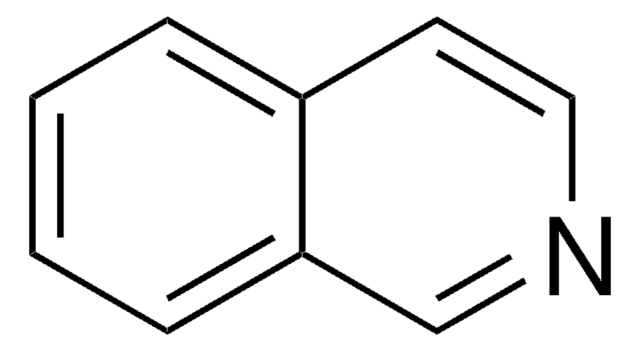
Quinazoline
99%
Synonym(s):
1,3-Benzodiazine, Benzopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H6N2
CAS Number:
Molecular Weight:
130.15
Beilstein:
109370
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
bp
243 °C (lit.)
mp
46-48 °C (lit.)
solubility
H2O: freely soluble
organic solvents: soluble
SMILES string
c1ccc2ncncc2c1
InChI
1S/C8H6N2/c1-2-4-8-7(3-1)5-9-6-10-8/h1-6H
InChI key
JWVCLYRUEFBMGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Quinazolines has applications in medicinal chemistry due to their antibacterial, antifungal, anticonvulsant, anti-inflammatory and antitumor activities. It is the basic structural unit of pharmaceuticals and plays an important role in modern synthesis of antitumor drugs.
Application
Quinazoline was used to study the electrochemical behaviour of quinazoline using modern polarographic and voltammetric methods.
Biochem/physiol Actions
Genotoxicity of quinazoline was established by bacterial SOS Chromotest (Escherichia Coli).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reddy Amala et al.
BioImpacts : BI, 11(1), 15-22 (2021-01-21)
Introduction: Inflammation is the primary response caused due to harmful stimuli which are followed by the increased draining of plasma and immune cells from the body into the site of the injured tissue. A signaling cascade of growth factors and
Hamdoon A Mohammed
Medicinal chemistry (Shariqah (United Arab Emirates)), 16(8), 1044-1057 (2020-02-25)
Suaeda is a halophytic genus belonging to the Amaranthaceae family and can survive in the high salted marsh areas of the world. Suaeda plants can biosynthesize natural substances with powerful antioxidant activity and are considered as a renewable source of
Polarographic and voltammetric determination of quinazoline-the structural unit of anticancer drugs.
Hladikova J, et al.
Sensing in Electroanalysis, 3, 165-175 (2008)
Bruna Possato et al.
Dalton transactions (Cambridge, England : 2003), 46(24), 7926-7938 (2017-06-13)
We report on the investigation of a new series of symmetric trinuclear ruthenium complexes combined with azanaphthalene ligands: [Ru
Kunal Nepali et al.
European journal of medicinal chemistry, 196, 112291-112291 (2020-04-24)
This study reports the design, synthesis and evaluation of a series of histone deacetylase (HDAC) inhibitors containing purine/purine isoster as a capping group and an N-(2-aminophenyl)-benzamide unit. In vitro cytotoxicity studies reveal that benzamide 14 suppressed the growth of triple-negative breast
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service