116416
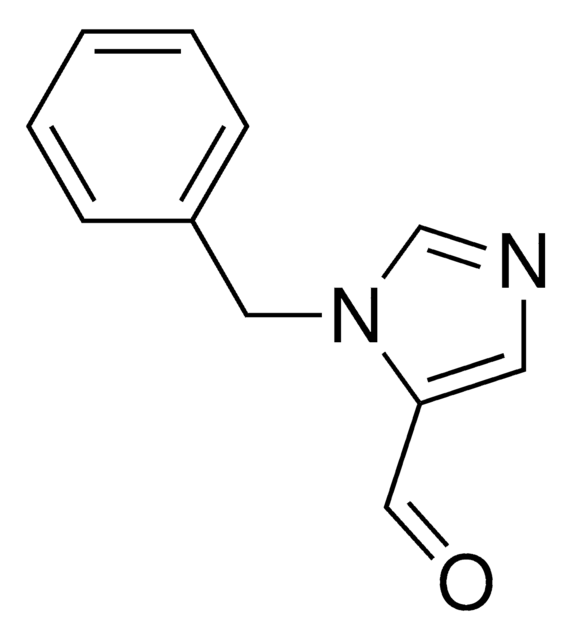
1-Benzylimidazole
99%
Synonym(s):
1-(Phenylmethyl)-1H-imidazole, 1-Benzyl-1H-imidazole, N-Benzylimidazole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C10H10N2
CAS Number:
Molecular Weight:
158.20
Beilstein:
114571
EC Number:
MDL number:
UNSPSC Code:
12352005
eCl@ss:
39161001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
310 °C (lit.)
mp
68-70 °C (lit.)
functional group
phenyl
SMILES string
C(c1ccccc1)n2ccnc2
InChI
1S/C10H10N2/c1-2-4-10(5-3-1)8-12-7-6-11-9-12/h1-7,9H,8H2
InChI key
KKKDZZRICRFGSD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Benzylimidazole has been used to prepare cyclodextrin-ionic liquid polymer (βCD-BIMOTs-TDI).
Biochem/physiol Actions
1-Benzylimidazole is a CYP inhibitor that inhibits the biotransformation of MeO-BDEs (methoxylated-brominated diphenyl ethers) to OH-BDEs (hydroxylated) in fishes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Grothusen et al.
Archives of toxicology, 71(1-2), 64-71 (1996-01-01)
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic hetero-atoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic
P Rothenbach et al.
Journal of applied physiology (Bethesda, Md. : 1985), 83(2), 530-536 (1997-08-01)
This study examines the hypothesis that intestinal ischemia-reperfusion (I/R) injury contributes to renal dysfunction by altered renal eicosanoid release. Anesthetized Sprague-Dawley rats underwent 60 min of sham or superior mesenteric artery (SMA) occlusion with 60 min of reperfusion. The I/R
K V Dileep et al.
International journal of biological macromolecules, 170, 415-423 (2020-12-30)
Alzheimer's disease (AD), a common chronic neurodegenerative disease, has become a major public health concern. Despite years of research, therapeutics for AD are limited. Overexpression of secretory glutaminyl cyclase (sQC) in AD brain leads to the formation of a highly
Jérémie Doiron et al.
European journal of medicinal chemistry, 46(9), 4010-4024 (2011-06-28)
A series of bis- and mono-benzonitrile or phenyl analogues of letrozole 1, bearing (1,2,3 and 1,2,5)-triazole or imidazole, were synthesized and screened for their anti-aromatase activities. The unsubstituted 1,2,3-triazole 10a derivative displayed inhibitory activity comparable with that of the aromatase
Muggundha Raoov et al.
Journal of hazardous materials, 263 Pt 2, 501-516 (2013-11-16)
Cyclodextrin-ionic liquid polymer (βCD-BIMOTs-TDI) was firstly synthesized using functionalized β-Cyclodextrin (CD) with 1-benzylimidazole (BIM) to form monofunctionalized CD (βCD-BIMOTs) and was further polymerized using toluene diisocyanate (TDI) linker to form insoluble βCD-BIMOTs-TDI. SEM characterization result shows that βCD-BIMOTs-TDI exhibits macropore
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)