116238
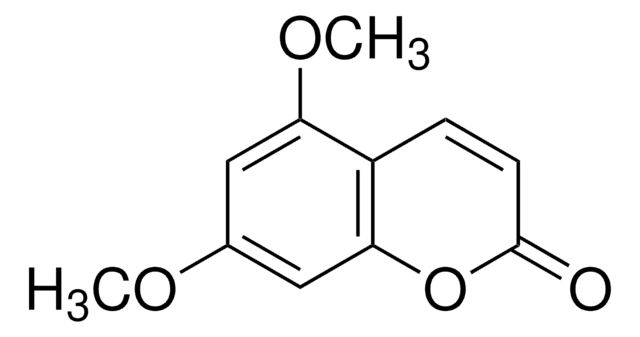
5,7-Dimethoxycoumarin
98%
Synonym(s):
Citropten, Limettin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H10O4
CAS Number:
Molecular Weight:
206.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
146-149 °C (lit.)
functional group
ester
SMILES string
COc1cc(OC)c2C=CC(=O)Oc2c1
InChI
1S/C11H10O4/c1-13-7-5-9(14-2)8-3-4-11(12)15-10(8)6-7/h3-6H,1-2H3
InChI key
NXJCRELRQHZBQA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5,7-dimethoxycoumarin is isolated and identified from leaves and fruits of Pelea anisata H. Mann, a plant whose fruit are used in the construction of mohikana leis. It induces frameshift mutagenesis in bacteria. It also causes lethal photosensitization and the formation of sister chromatid exchanges in Chinese hamster cells.
Biochem/physiol Actions
5,7-Dimethoxycoumarin induces the processes of differentiation and melanogenesis in murine (B16) and human (A375).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniela Alesiani et al.
International journal of oncology, 34(6), 1727-1735 (2009-05-09)
In this study, the processes of differentiation and melanogenesis induced by 5,7-dimethoxycoumarin in murine (B16) and human (A375) melanoma cells were investigated. Taking into account the previously demonstrated antiproliferative and differentiation activities of this compound, we examined Ras/Raf/Mek/Erk mitogen-activated protein
Takeshi Kinoshita et al.
Chemical & pharmaceutical bulletin, 50(1), 118-120 (2002-02-05)
A new C-8 prenylated 5,7-dimethoxycoumarin named omphamurrayin was isolated from the leaves of Murraya paniculata var. omphalocarpa, and its structure was established as 5,7-dimethoxy-8-(1-oxo-2-senecioyl-3-methyl-3-butenyl)-2H-1-benzopyran-2-one on the basis of the spectroscopic evidence. The taxonomic status of M. paniculata var. omphalocarpa is
S Makki et al.
Journal of chromatography, 563(2), 407-413 (1991-02-15)
Citropten (5,7-dimethoxycoumarin) and bergapten (5-methoxypsoralen) are present in bergamot oil which is used as a tanning cosmetic product. The aim of this study was to quantify, using high-performance liquid chromatography, the amount of citropten and bergapten in the skin after
V Jung et al.
Biochimica et biophysica acta, 740(1), 64-72 (1983-05-20)
The photobinding of 5,7-dimethoxycoumarin to isolated adenovirus-type 2 DNA has been investigated with respect to the influence of the ionic environment, and varying molar ratios of DNA(p): 5,7-dimethoxycoumarin. In particular, the ultraviolet radiation-induced covalent addition of 5,7-dimethoxycoumarin to adenovirus DNA
Isolation and characterization of the photoadducts of 5,7-dimethoxycoumarin and adenosine.
T H Cho et al.
Photochemistry and photobiology, 46(2), 305-309 (1987-08-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service