107425
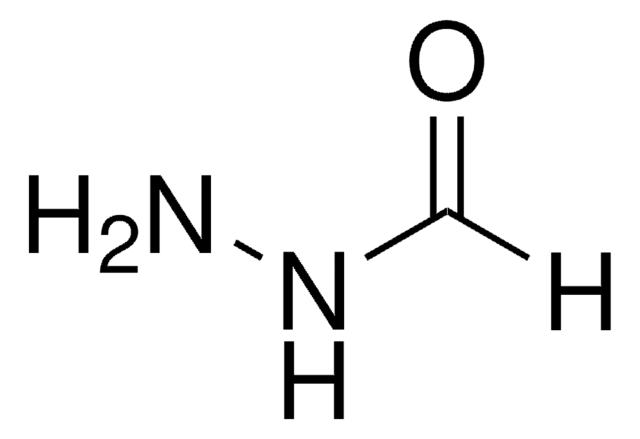
Nicotinic hydrazide
97%
Synonym(s):
Nicotinic acid hydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H7N3O
CAS Number:
Molecular Weight:
137.14
Beilstein:
119299
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
159-161 °C (lit.)
solubility
H2O: soluble 50 mg/mL
functional group
amine
SMILES string
NNC(=O)c1cccnc1
InChI
1S/C6H7N3O/c7-9-6(10)5-2-1-3-8-4-5/h1-4H,7H2,(H,9,10)
InChI key
KFUSANSHCADHNJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Nicotinic hydrazide is a heterocyclic compound that can be used to synthesize Schiff bases.
Application
Nicotinic hydrazide was used in hydrazone library formation. It was used to study the oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase.
Biochem/physiol Actions
Nicotinic hydrazide is an inhibitor of peroxidase enzyme. It forms solid metal complexes having strong biological activity.
Preparation Note
Nicotinic hydrazide dissolves in water at a concentration of 50 mg/ml to form a clear, colourless solution.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Goral et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1347-1352 (2001-02-15)
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension
H A Shoeb et al.
Antimicrobial agents and chemotherapy, 27(3), 399-403 (1985-03-01)
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH
Thermo-chemical behavior of solid nicotinic hydrazide metal complexes in correlation with their stoichiometry.
Sekkina MM and El-Azm MG.
Thermochimica Acta, 77(1), 211-218 (1984)
Synthesis, spectroscopic characterization, and crystal structures of Schiff bases derived from nicotinic hydrazide
Diouf F, et al.
IOSR journal of applied chemistry, 1 serie II (2022)
B J van der Walt et al.
The International journal of biochemistry, 26(9), 1081-1093 (1994-09-01)
1. Superoxide was generated during the auto-oxidation of the antituberculous drug, isonicotinic acid hydrazide (INH), but not with its meta-isomer, nicotinic acid hydrazide (NH). During Fe(3+)-stimulated oxidation of INH and NH, aromatic hydroxylation occurred which was inhibited by the chelating
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service