103918
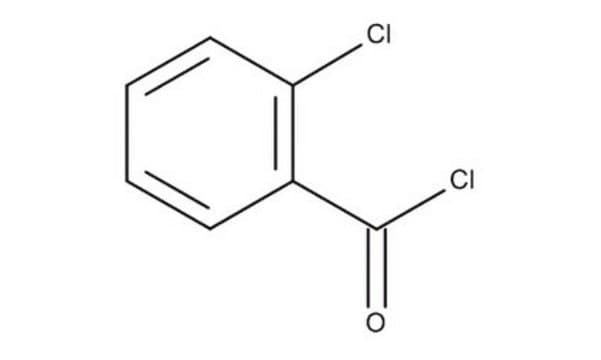
2-Chlorobenzoyl chloride
95%
Synonym(s):
o-Chlorobenzoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4COCl
CAS Number:
Molecular Weight:
175.01
Beilstein:
386435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.572 (lit.)
bp
238 °C (lit.)
mp
−4-−3 °C (lit.)
density
1.382 g/mL at 25 °C (lit.)
SMILES string
ClC(=O)c1ccccc1Cl
InChI
1S/C7H4Cl2O/c8-6-4-2-1-3-5(6)7(9)10/h1-4H
InChI key
ONIKNECPXCLUHT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Chlorobenzoyl chloride reacts with aromatic amines and ammonium thiocyanate using polyethylene glycol-400 as the catalyst under the condition of solid-liquid phase-transfer catalysis to form N-aryl-N′(2-chlorobenzoyl) thioureas. 2-Chlorobenzoyl chloride causes the acylation of polystyrene during the preparation and regeneration of the polystyrene-based resin.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
255.2 °F - closed cup - DIN 51758
Flash Point(C)
124 °C - closed cup - DIN 51758
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Simple and efficient synthesis of 2-chlorotritylchloride resin.
Orosz G and Kiss L.
Tetrahedron Letters, 39(20), 3241-3242 (1998)
Phase-transfer-catalyzed synthesis of N-aryl-N'-(2-chlorobenzoyl)-thiourea derivatives.
Zhang Y, et al.
Synthetic Communications, 27(5), 751-756 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service